Introduction
Breast cancer remains a significant global health challenge, primarily due to its high incidence and the complexity of treatments required [1, 2]. It is among the most frequently diagnosed cancers worldwide [1]. The hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER-2–) subtype accounts for approximately 70% of all breast cancer cases [3]. Despite advancements in endocrine therapy (ET), which is fundamental in managing HR+/HER-2– advanced breast cancer (ABC), the emergence of resistance presents a substantial barrier, often necessitating a transition to more toxic therapies, such as chemotherapy or, more recently, antibody-drug conjugates [2].
The introduction of CDK4/6 inhibitors, such as palbociclib, ribociclib, and abemaciclib, has markedly transformed therapeutic strategies for HR+/HER-2– ABC [4–6]. Combined with ET, these agents have shown significant improvements in progression-free survival and overall survival, leading to their recommendation as preferred first-line options by international guidelines [2, 7, 8]. However, while controlled clinical trials have demonstrated the efficacy and safety of CDK4/6 inhibitors, there is a growing need for real-world evidence to assess treatment patterns, outcomes, and adherence to guidelines in routine clinical settings. This need is important in diverse healthcare systems where treatment accessibility and differences in guideline adoption may influence clinical decisions and treatment results [9, 10].
This study addresses this knowledge gap by conducting a retrospective analysis of real-world treatment patterns for HR+/HER-2– ABC in Poland. Since September 2019, the CDK4/6 inhibitors have become accessible to first-line treatment of HR+/HER-2– ABC patients in Poland [11]. Notably, as early as 2018, before the widespread availability of CDK4/6 inhibitors, the guidelines of the Polish Society of Clinical Oncology recommended their use in both first- and second-line treatment for HR+/HER-2– ABC [12]. By examining data on treatment patterns, patient demographics, and clinical characteristics, this study aims to evaluate the consistency of clinical practices with established guidelines and identify trends that may guide future treatment strategies. Given the observed rising breast cancer mortality rates in Poland [13] and disparities in survival rates compared with other European countries [14], a deeper understanding of current clinical approaches to HR+/HER-2– ABC management can have significant implications for both patient care and health policy development.
Material and methods
The HABER study (HR+/HER-2– ABC – trEatment patteRns in Poland) was a multicentre, retrospective, non-interventional review of treatment patterns among patients with ABC commencing first-line therapy between September 2020 and August 2021. The trial was registered with ClinicalTrials.gov, NCT05478590.
The primary objective of the HABER study was to assess treatment patterns of HR+/HER– ABC patients over one year and evaluate their alignment with national [12, 15] and international guidelines [2, 7, 16, 17]. Other objectives included the characterization of the population and evaluation of relations between ABC treatment types and patient demographics and characteristics, including concomitant therapies, previous use of neoadjuvant and adjuvant therapies, and time to relapse from early breast cancer.
Data sources
The study utilized electronic medical records from 17 clinical sites across Poland, comprising seven large and 11 smaller sites. The size of the centres was defined during the feasibility process based on the declared number of patients with HR+/HER-2– ABC treated in the first line and the contract volume from the payer. Data collected included patient demographics, menopausal status, comorbidities, and clinical and treatment characteristics. Medical records were reviewed for specific information on cancer staging, metastatic sites, the presence of visceral crisis, and comorbidities that could potentially influence treatment choices. Data entry was conducted by medical professionals with expertise in ABC management using the GoInsights™ electronic case report form platform (2KMM sp. z o.o., Katowice, Poland). To ensure data integrity and compliance, validations were implemented, and re-entry checks were conducted on randomly selected data entries. The study did not require additional patient visits or follow-ups. All collected data were de-identified. The study did not require collecting patient consent. This study did not require approval from an ethics review board.
Patients
Patients were eligible if they met the following criteria: age of 18 years or older, diagnosis of ABC, documented HR+ (estrogen and/or progesterone receptors) without HER-2 receptor overexpression or HER2 gene amplification, no prior treatments for advanced or metastatic disease, absence of symptomatic central nervous system metastases or other malignancies requiring active treatment, and non-participation in any other clinical trial. All patients had to begin ABC therapy in the eligibility period of one year, between 1 September 2020 and 31 August 2021.
Variables
Therapeutic approaches used as the first-line treatment for HR+/HER-2– ABC included single-agent or combination chemotherapy, ET, and CDK4/6 inhibitors combined with ET. The following variables were extracted for all patients enrolled: age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, menopausal status, age at diagnosis, cancer stage at diagnosis, including previous diagnosis of early breast cancer, previous therapy for early breast cancer, and relapse-free interval (RFI), prescribed therapy, location of metastases (bones, lymph nodes, central nervous system, and visceral metastases including liver, lung, adrenal glands, and gastrointestinal tract) and presence of visceral crisis. Relapse-free interval was defined as the time between the end of adjuvant treatment of early breast cancer and the diagnosis of ABC.
Statistical analysis
The analysis in this study was exploratory, reflecting its non-interventional nature, with no hypothesis testing or inferential statistical methods employed. Descriptive statistics were used to summarize treatment patterns and patient characteristics. The sample size, mean, and standard deviation (SD) were reported for numeric variables. For categorical variables, the sample size, count, and percentage were provided for each category. No imputation for missing values was performed. The distributions among characteristics were assessed by the χ2 test and the Welch’s t-test. All analyses were conducted using the validated version of the R Statistical Package, version 3.6.3.
The results are intended to be interpreted cautiously. They are not designed to support causal inferences.
Results
Overall, 459 patient records were initially identified, with 19 (4.2%) excluded due to failure to meet inclusion criteria, duplication of records, or lack of treatment for ABC. The final cohort comprised 440 women (Table 1).
Table 1
Patient characteristics
The mean age was 62.7 years, with a substantial proportion (80.9%) aged ≥ 50 years. The majority of patients (80.4%) presented with the ECOG performance status of 0 or 1. At least one comorbidity was present in 65% of patients, with hypertension (29.8%), diabetes (10.5%), and peripheral vascular disease (8.4%) being the most prevalent (Table 1).
Most patients (94.1%) had metastatic (vs. locally advanced) disease, with bone and visceral metastases being the most frequent sites of metastasis (Table 1). Previous diagnosis of early breast cancer was documented for 51.4% of patients, the majority of whom had received adjuvant ET before the diagnosis of ABC (Table 1). The mean ± SD RFI was 19.4 ±34.9 months. Most patients (328/440, 74.5%) were treated at large clinical centres.
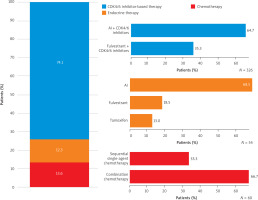
CDK4/6 inhibitor-based therapy was used in 326/440 pa- tients (74.1%) (Figure 1). All patients aged ≤ 40 years (n = 21) received CDK4/6 inhibitor-based therapy. Across the decades of age at ABC diagnosis, the proportion of patients receiving ET alone increased, ranging from 6.8% in patients aged 41–50 years to 47.1% in those aged ≥ 81 years. In both large and smaller clinical centres, the majority of patients were treated with CDK4/6 inhibitor-based combinations (78.4% and 61.7%, respectively). In the small clinical sites, chemotherapy was used more often than ET (25.9% vs. 12.5%), while ET alone prevailed over chemotherapy in larger sites (12.2% vs. 9.5%) (Table 2). Combinations of CDK4/6 inhibitors with aromatase inhibitors (AI) were more common than with fulvestrant (64.7% vs. 35.3%, p < 0.0001). Overall, twice more patients received combination chemotherapy than single-agent sequential chemotherapy (Figure 1).
Table 2
Clinical setting and disease characteristics according to HR+/HER– advanced breast cancer treatments
Table 2 presents first-line treatment classes for HR+/HER– ABC according to disease characteristics. CDK4/6 inhibitor-based combinations were used frequently in patients with ECOG performance status 0–1 and previous diagnosis of early breast cancer (Table 2). The mean RFI ± SD was 25.5 ±52.9 months in patients treated with CDK4/6 inhibitor-based therapies; however, it was numerically longer in those patients receiving CDK4/6 inhibitor combination with AI (35.3 ±67.7 months) than with fulvestrant (16.5 ±31.7 months). Patients receiving singe-agent chemotherapy had longer mean RFI (28.2 ±52.8 months) than those treated with combination chemotherapy (9.8 ±17.5 months). In the ET group, the mean RFI was 15.2 ±40.2 months.
Overall, CDK4/6-treated patients were younger (mean age ± SD, 61.3 ±11.7 years) than those receiving ET (mean age ± SD, 71.8 ±11.6 years), p < 0.0001, and of similar age to those receiving chemotherapy (mean age ± SD, 62.0 ±13.2 years), p = 0.6904. Most patients diagnosed with visceral crisis were treated with chemotherapy (Table 2). Patients with the ECOG performance status ≥ 2 were more frequently treated with ET or chemotherapy than CDK4/6 inhibitor-based combinations (Table 2).
Discussion
The present analysis characterizes the demographic and disease profiles, as well as treatment patterns, of a real- world population of Polish patients with HR+/HER-2– ABC. Patients within the HABER study were predominantly postmenopausal, had a favourable performance status, and two-thirds had at least one comorbidity, most often hypertension. A substantial proportion of these patients received CDK4/6 inhibitor-based therapies in the first-line setting, reflecting the evolution of the treatment landscape by expanding the therapeutic armamentarium with targeted therapies. The guidelines recommend CDK4/6 inhibitors combined with ET, either an AI or fulvestrant, as the standard treatment for patients with HR+/HER– ABC [2, 7]. These combinations have demonstrated significant survival benefits while maintaining or improving patients’ quality of life in both de novo metastatic and recurrent breast cancer cases [2, 7, 18]. Notably, CDK4/6 inhibitor combinations with ET were the most commonly utilized treatment options across large and smaller clinical centres, indicating increased adoption of local [12, 15], European [2, 7], and international guidelines [8]. Historically, ET and chemotherapy were the predominant treatments, with varying patterns observed across Europe and the United States [19]. In 2017, during the early phase of this pivotal shift in care, 10% of patients with HR+/HER-2– ABC received CDK4/6 inhibitors in combination with ET [20]. The SONABRE Registry documented a significant increase in the use of CDK4/6 inhibitor-based therapies, with 54% of patients diagnosed with ABC between 2017 and 2019 receiving these treatments within the first three years of diagnosis. Reported improvements in overall survival were partially attributed to the integration of targeted therapies into clinical practice in the Netherlands [21].
Albeit age is not a decisive factor in selecting first-line treatment for patients with ABC [2], in the HABER study, patients receiving ET were older compared to those treated with CDK4/6 inhibitor-based therapies or chemotherapy. The study did not identify the patient’s age as a limiting factor in applying any therapy, i.e., in every age category, there were patients treated with drugs from every category, except the youngest patients treated solely with CDK4/6 inhibitor-based combinations. Key determinants of treatment decisions include performance status and the presence of comorbidities [2]. In our study, patients with ECOG performance scores ≥ 2 were more likely to receive ET or chemotherapy rather than CDK4/6 inhibitor-based therapies. Clinical considerations, such as the need for a low-toxicity option or the requirement for a rapid therapeutic response, further influence the selection between ET and chemotherapy. Additionally, organizational factors, such as meeting the requirements of drug access programs, e.g., additional time required for patient evaluation during qualification for the treatment, may delay the initiation of treatment or influence decisions about treatment type [11]. Importantly, choosing a treatment other than the recommended first-line option does not exclude the use of CDK4/6 inhibitor-based therapies in subsequent lines [22]. Evidence from both randomized trials and real-world data in Poland has shown no significant differences in survival outcomes between the use of CDK4/6 inhibitors combined with ET in the first-line vs. second-line settings [23, 24]. Identifying predictive factors of response will be essential to optimize the sequencing of therapies and tailor treatment strategies for individual patients. However, nowadays in Poland, most patients are treated with CDK4/6 inhibitor-based therapies in the first line [24].
The CDK4/6 inhibitors were combined more frequently with AI than with fulvestrant, in line with earlier reports describing Polish real-world practice of HR+/HER– ABC treatment within the drug access program [11, 24]. Aromatase inhibitors in combination with a CDK4/6 inhibitor are preferred when a patient did not relapse on AI or relapsed ≥ 12 months after stopping adjuvant AI; otherwise, fulvestrant is recommended [2, 12]. The difference in the mean time to relapse from adjuvant therapy confirms that this recommendation is followed in studied real-world practice. Nordic experts reached a consensus that CDK4/6 inhibitor combination with fulvestrant can be justified in the disease-free interval from early breast cancer to ABC < 2 years [25]. All CDK4/6 inhibitors were studied in combination with fulvestrant, and trials included patients with early relapse after completion of (neo-)adjuvant treatment, demonstrating a progression-free survival advantage [26–28]. The observed difference in the utilization of AI and fulvestrant with CDK4/6 inhibitors may also come from the fact that a significant proportion of patients in our study were diagnosed with de novo metastatic disease.
CDK4/6 inhibitor-based therapies were the preferred treatment across all metastatic locations, including the bones, lymph nodes, and visceral organs. Notably, in this study, only a small number of patients presented with asymptomatic brain metastases, all of whom were treated with CDK4/6 inhibitors combined with ET. Patients with metastases in two or more visceral organs, however, received CDK4/6 inhibitor-based therapy less frequently compared to those with other metastatic patterns. In this case, chemotherapy was more frequently selected. Combination chemotherapy was twice as frequently used than sequential single-agent chemotherapy, possibly explained by a higher proportion of those progressing rapidly with life-threatening visceral metastases or in need of rapid symptom/disease control among patients treated. Chemotherapy remained a relevant choice upon diagnosis of visceral crises [29].
In our study, almost half of the patients had de novo metastatic breast cancer, which was relatively high compared to other series [30, 31]. This high prevalence of metastatic disease at diagnosis may be a consequence of the poor performance of the national breast cancer screening program, which at the time of the study was targeting women aged 50–69 years old – the population coverage of the screening program was only 34.2% [32]. Access to breast cancer screening programs aimed at early detection and treatment is fundamental [14], and in 2023, the program’s eligibility age was expanded to women aged 45–74 years.
This study has several limitations. The small size of the analysed patient subgroups, along with the lack of preparation for statistical hypothesis testing, increases the susceptibility to bias in statistical significance testing. As a result, the findings should be interpreted as indicative of potential trends rather than definitive conclusions. The HABER study did not evaluate treatment outcomes. The study did not capture changes in biomarker status. Because patient characteristics influenced the selection of first-line treatments, direct comparisons of survival outcomes between groups have limited value. The extensive granulation of data on comorbidities within the studied population did not allow for conclusions regarding their influence on treatment choices.
Conclusions
CDK4/6 inhibitor-based therapy was the most commonly chosen treatment, reflecting significant integration of evidence-based guidelines into clinical practice in Poland. The observed treatment patterns closely aligned with current recommendations for managing HR+/HER-2– ABC. Chemotherapy was administered primarily in cases of rapid tumour progression or immediate life-threatening visceral crises, consistent with established practices. Variations in treatment patterns underscored the individualization of therapy, influenced by factors such as patient performance status and the time elapsed until the relapse from early breast cancer.