A 26-year-old male patient with a history of tetralogy of Fallot (TOF) presented for a routine outpatient visit. The patient had undergone multiple prior cardiac procedures, including a Blalock-Taussig shunt (1997), complete surgical repair with a transannular patch (1999), pulmonary valve replacement with a 27-mm Sorin Crown (2018), and repair of a pulmonary artery pseudoaneurysm (PAP) following bovine pericardial patch detachment (2020).
Despite reporting stable well-being during follow-up, transthoracic echocardiography (TTE) revealed new pathological findings. Color Doppler imaging identified eccentric jet flow originating above the valve annulus, directed along the sinus of Valsalva towards the interventricular septum (Figures 1 A, B). Additionally, the right ventricular outflow tract (RVOT) was aneurysmally dilated, with a pressure gradient of 41/26 mm Hg and severe pulmonary valve regurgitation.
Figure 1
Image of the recurrent pseudoaneurysm of pulmonary artery in transthoracic echocardiography (TTE) and computed tomography (CT). Panels A and B show images from TTE with additional eccentric echoes in color Doppler originating above the level of the valve annulus, directed along the sinus of Valsalva to the interventricular septum. Panels C and D show axial and sagittal planes from CT of the right ventricle outflow tract (RVOT). Panel E contains 3D reconstruction of RVOT – yellow arrow shows reconstructed cavity of pulmonary artery pseudoaneurysm, blue arrow – level of pulmonary valve, gray arrow – subvalvular RVOT, white arrow – pulmonary branches. Panel F shows 3D reconstruction of patient’s heart with marked pseudoaneurysm (white arrow)
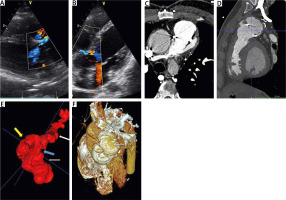
Computed tomography (CT) showed a wide RVOT (45 × 36 mm) at the subvalvular level, a dilated PV annulus (31 × 30 mm), a large 41 × 40 mm pulmonary trunk aneurysm, and irregular sudden dilatation of its walls, which may correspond to pseudoaneurysm (Figures 1 C–F). Additional, fissure-like paravalvular leak (about 2 mm wide) was visualized, which probably corresponded to the unexpected jet with to-and-fro flow observed on TTE.
After multidisciplinary evaluation by the Heart Team, the patient was scheduled for urgent surgical intervention. While awaiting surgery at home (2-week period), the patient remained clinically stable. Surgical repair was successfully performed using a 25-mm Biointegral valved conduit.
At the 4-month postoperative follow-up, TTE demonstrated favorable outcomes, with a low pressure gradient across the pulmonary bioprosthesis (17/10 mm Hg) and only trace regurgitation.
PAP is a rare finding, but potentially life-threatening [1, 2]. Risk factors include iatrogenic, trauma, infectious diseases, vasculitis, cancer, congenital diseases and pulmonary hypertension [1, 2]. It is suggested that hemoptysis is the most common symptom. However, symptoms may range from completely asymptomatic to dyspnea, coughing or even sudden death [1–5]. Interestingly, PAP is a rare complication even among patients with congenital heart defects [3, 4]. In a study conducted at Boston Children’s Hospital, it was observed in only 2.1% (20/972) among patients after surgical right ventricle–to–pulmonary artery conduit placement over a 20-year period [3]. The authors stated that younger age, smaller size, TOF, pulmonary homograft conduit, presence of unrestrictive ventricular septal defect after conduit placement, and at least systemic right ventricular pressure may be additional risk factors of PAP [3].
This case highlights the importance of careful, long-term surveillance of patients with complex congenital heart disease.






