Introduction
The EUROCARE-5 population study based on 107 oncological registers in 29 European countries for the period from 1999 to 2007 reported a 37.6% 5-year survival rate of ovarian cancer in all stages of the disease [1]. According to the CONCORD-2 program based on cancer registers in 61 countries, the 5-year survival rate for ovarian carcinoma in the advanced stage was 30%, and it was significantly lower compared to the disease limited to the pelvis, in which case the survival rate reached 80% [2]. Advanced ovarian carcinoma (AOC) remains a challenge for surgeons due to the need for complete surgical extirpation of the tumor with the purpose of extension of the period of survival. The standard surgical intervention, according to the guidelines, remains the total abdominal hysterectomy (TAH) with bilateral salpingo-oophorectomy and omentectomy [3]. Radical pelvic surgery, including hysterectomy, became necessary for a significant proportion of patients. A surgical technique was described in 1968 by Hudson and Chir, in which the retroperitoneal approach to the pelvic structures allows the complete removal of the neoplasm, without it being resected and without the persistence of residual tumor [4]. The procedure includes en bloc extirpation of the uterus with both adnexa, pelvic peritoneum with or without resection of the rectosigmoid colon and partial peritonectomy. The surgical technique for the retroperitoneal approach is similar to that for radical hysterectomy for cervical cancer. This type of hysterectomy can be termed extended hysterectomy (EH). Our study aims to assess the significance of EH for the overall and relapse-free survival rate.
Material and methods
The data were collected retrospectively from the hospital records of 104 patients operated on in the period from 2004 to 2012 at the Clinic of Gynecology of General Hospital for Active Treatment „Saint Anna” – Varna City for the treatment of AOC (FIGO stages II-IV). The patients were analyzed with respect to the age, FIGO stage, histological variant and grading of tumor, size and location of the residual tumor, and types of hysterectomy – TAH, EH, and other surgical procedures. The staging was implemented according to the FIGO classification from 1998. 95% of patients received adjuvant chemotherapy in six cycles of combined chemotherapy with carboplatin (in a dose calculated by area under concentration-time curve [AUC] of 5-7.5) and paclitaxel of 175 mg/m2 administered once every three weeks or with an alternative regimen – combination of paclitaxel of 135 mg/m2 on day 1 and 60 mg/m2 on day 8 with cisplatin of 75-100 mg/m2 once every three weeks. Neoadjuvant chemotherapy was administered to a minority of patients with paclitaxel of 175 mg/m2 and carboplatin (AUC of 6-5). Patients were monitored during 60-120 months. Information about their live status was obtained from the National cancer register.
The data from the study were entered and analyzed with the statistical software MedCalc2018. Descriptive analysis was applied for the determination of an average value, standard deviation, and median. The curves of survival rate were drawn by the method of Kaplan-Meier and were estimated with the log-rank test. The factors and differences with the level of significance of < 0.05 (p < 0.05) in all analyses were considered statistically significant.
Results
The characteristics of the population of patients are summarized in Table 1.
Table 1
Characteristics of the population of patients
Among 23 patients (22.1%) TAH with bilateral adnexectomy and omentectomy were performed. In 74 patients (71.2%) EH with a retroperitoneal approach and some degree of peritonectomy with omentectomy were performed; only in six patients did the procedure include rectosigmoid resection. The two groups – with TAH and with EH – were juxtaposed and analyzed with respect to the overall survival and disease-free survival. Seven patients underwent palliative or diagnostic surgical procedures without eradication of the disease. The latter group was not included in the survival analysis. Table 2 shows comparative data for the group with TAH and the group with EH.
Table 2
Comparison of groups with total and extended hysterectomy in relation to FIGO stage, residual tumor, grading, histological variant of tumor, and type of chemotherapy
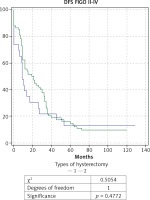
The analysis of survival rate (Figs. 1 and 2), which covered patients of FIGO stages II-IV, did not demonstrate a statistically significant difference in the overall survival (OS), and disease-free survival (DFS) between the two groups: with TAH (n = 23) and EH (n = 74) (48.4 months vs. 47.6 months for OS, and 27.7 months vs. 33.3 months for DFS).
Fig. 1
Kaplan-Meier curve for overall survival of patients with ovarian carcinoma of FIGO stages II-IV, divided into two gro- ups according to type of hysterectomy: group 1 with total abdominal hysterectomy (TAH), and group 2 with extended hysterectomy (EH)
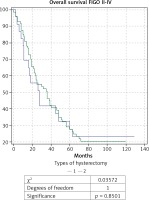
Fig. 2
Kaplan-Meier curve for disease-free survival (DFS) of patients with ovarian carcinoma of FIGO stages II-IV, divided into two groups according to type of hysterectomy: group 1 with total abdominal hysterectomy (TAH), and group 2 with extended hysterectomy (EH)
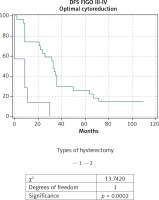
When comparing the EH (n = 69) and TAH (n = 15) in patients with AOC of stages III and IV a significantly higher OS rate, DFS rate, and 5-year survival rate were observed in the group of EH: respectively 44.7 months; 29 months and 26% compared to 22.1 months; 10.4 months and 7% for TAH. The comparative Kaplan-Meier curves for the overall and disease-free survival rates for the subgroups with FIGO stages III-IV are shown in Figure 3 and Figure 4. We evaluated the survival rate in the subgroup of patients with FIGO III-IV and residual tumor of 0-1 cm to reduce the burden of the latter as a major predictor. A significant difference in the overall and relapse-free survival rate was also found in the subpopulation of patients with FIGO III-IV and an optimal cytoreduction (Figs. 5 and 6). The OS rate, DFS rate, and 5-year rate were respectively: 66.4 months; 41.3 months; 52% for EH (n = 27) and 17.7 months; 8.4 months and 0% for TAH (n = 7).
Fig. 3
Kaplan-Meier curve for overall survival rate of patients with ovarian carcinoma of FIGO stage III-IV, divided into two groups according to type of hysterectomy: group 1 with total abdominal hysterectomy (TAH), and group 2 with extended hysterectomy (EH)
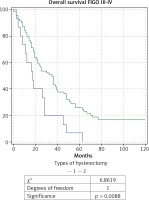
Fig. 4
Kaplan-Meier curve for disease-free survival (DFS) rate of patients with ovarian carcinoma of FIGO stage III-IV, divided into two groups according to type of hysterectomy: group 1 with total abdominal hysterectomy (TAH), and group 2 with extended hysterectomy (EH)
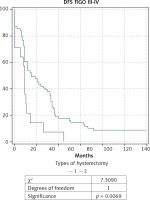
Fig. 5
Kaplan-Meier curve for overall survival rate of patients with ovarian carcinoma of FIGO stages III-IV, and achieved op- timal cytoreduction, divided into two groups according to type of hysterectomy: group 1 with total abdominal hysterectomy (TAH), and group 2 with extended hysterectomy (EH)
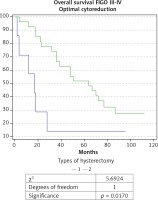
Fig. 6
Kaplan-Meier curve for disease-free survival (DFS) rate of patients with ovarian carcinoma of FIGO stages III-IV and achie- ved optimal cytoreduction, divided into two groups according to type of hysterectomy: group 1 with total abdominal hysterecto- my (TAH), and group 2 with extended hysterectomy (EH)
Discussion
Optimal cytoreduction in the case of AOC can be achieved employing TAH with bilateral adnexectomy, and omentectomy. In our cohort, TAH was sufficient to achieve optimal cytoreduction for only 13 patients out of 104. Five-year survival was observed in only 6 of them, who were in FIGO stage II, and comprised about 50% of FIGO stage II of our sample. Ovarian carcinoma of FIGO stage II is hard to define and is found in about 10% of all cases [5]. Stage II, according to the FIGO classification of ovarian cancer, includes treatable tumors, tumors confined to the pelvis and metastatic tumors on the pelvic peritoneum, which usually have poorer prognosis [5]. Those facts show that the minimum standard requirement for surgical treatment of AOC would be sufficiently effective only for a small proportion of patients.
The EH, on the other hand, contributes to improvement of overall and disease-free survival rate in patients in FIGO stage III and IV. Many authors have evaluated the EH and its modifications in ovarian carcinoma. However, not all of them succeeded in proving explicitly its benefit for the survival rate [6]. Alleti et al. [7] compared the radical pelvic surgery with rectosigmoid resection, or peritoneal stripping with standard pelvic surgery in patients with AOC of FIGO stages IIIC and IV, and found that radical surgical efforts are connected with improvement of prognosis. They considered that the borders of the pelvic disease could be determined with difficulty in cases of extensive involvement of the pelvis, and there is a possibility for the residual tumor to be omitted. In their cohort of patients, they studied a subgroup with optimal cytoreduction, in which they found a statistically significant benefit of radical pelvic surgery for survival, underlining that the patients benefited from it in the cases of possible extirpation of the disease in the abdominal region as well [7]. The analysis of our data also demonstrated that the attempt for radicality in the pelvic region through EH with a retroperitoneal approach, with or without pelvic peritonectomy, or rectosigmoid resection brought about a significant benefit for the survival rate of patients with residual tumor of 0-1 cm.
The indications for performing of EH were summarized by Eisenkop in 1991 [8]. The two main indications are extensive confluent tumor involvement of the pelvis and rectouterine pouch, and the subjective surgeons’ assessment of the possibility of the complete removal of the disease using non-radical procedures. The clinical benefit of the EH is based on its demonstrated efficacy for the removal of the whole pelvic disease and its contribution for reduction of the size of residual tumor [9].
Once an optimal cytoreduction is achieved (residual tumor of 0-1 cm), the EH gives an advantage over the TAH in terms of survival. A probable reason for that is the greater radicality of the procedure with removal of macroscopically invisible (microscopic) tumor lesions, as it reduces the residual tumor burden and supports the subsequent chemotherapy. Furthermore, Pereira et al. – as well as our study – showed that it also removed an „invisible” residual tumor such as parametrial and vaginal invasion, when the tumor developed by direct extension [10]. These facts underlined the significance of the retroperitoneal approach and EH for the cytoreductive treatment and identification of metastatic disease.
The clinical benefit of the EH is based on its demonstrated efficacy for removal of the whole pelvic disease, and its contribution to the size of residual tumor [11]. Optimal cytoreduction is achievable in 74% to 100% of cases of EH, with almost complete clearance of the advanced pelvic tumor [7, 8, 11-17].
It appears that the successes of the EH for the optimal cytoreduction may not influence the clinical outcome in all patients with AOC, but it may be beneficial only where attempts for resection of disease in the upper abdomen are undertaken [6, 7]. Amongst our patients with an overall survival rate of up to one year after the operation, we found a significant percentage (67%) of high tumor dissemination (in 14 out of 21 patients), i.e., residual tumor in the upper abdomen (liver, diaphragm) despite the EH.
This study has some limitations and strengths. The limitations are the small number of patients and heterogeneous types of ovarian cancer. The strengths are that we present the 5-year survival rate and that one surgical team performed all procedures (the procedure is standardized). We are continuing with this study and with follow-up of the included patients and we will be ready with the 10-year survival rate soon.











