Introduction
Vitamin D deficiency is linked to different immunological disorders including rheumatoid arthritis, lupus erythematosus, and multiple sclerosis [1–3].
Additionally, vitamin D deficiency has been reported with autoimmune thyroid disorders (AITDs) [i.e. Hashimoto’s thyroiditis (HT), and Graves`s disease (GD)] [4, 5].
Vitamin D is fat-soluble, and it comes from exposure to the sun’s ultraviolet B-light (290–320 nm) [6]. Vitamin D enters the bloodstream linked to a vitamin D-binding protein, then it is hydroxylated to 25(OH)D and metabolized to generate the active metabolite calciferol [1,25- dihydroxy vitamin D (1,25(OH)2D)] [7]. The 25(OH)D has a half-life of 2–3 weeks and is the most prevalent circulating precursor of active vitamin D [8]. Serum 25(OH)D is the most extensively used biomarker to reflect and/or to measure serum vitamin D [8].
The serum 1,25(OH)2D level is not a reliable marker for vitamin D status because it is only decreased after severe vitamin D deficiency [9]. Additionally, it has a short half-life, and it is regulated by the parathyroid hormone (PTH), calcium, and phosphate [8]. The autoimmune thyroid disorders were discovered to be associated with vitamin D receptor gene polymorphisms [10]. So, this cross-sectional study was designed to detect the relationship between 25(OH)D and adolescent hypothyroidism.
Material and methods
A total of 180 adolescents were recruited for this cross-sectional research, which was conducted in West Kazakhstan (Aktobe) over 2 years (2021–2022) to detect the relationship between 25(OH)D and adolescent hypothyroidism.
Participants were included in the current study after approval (No. 10 dated 04.10.2020) from the Ethics Committee of West Kazakhstan Medical University, and after informed consent following the Helsinki Declaration from the participants themselves, and their guardians.
After thorough evaluation, including a thorough history, and clinical examination, the weight and height of the studied participants were measured to calculate the body mass index (BMI).
Inclusion criteria were adolescents (12–18 years old), with regular menstrual cycles, normal BMI 18.5– 24.9 kg/m2 [11], without any known chronic or endocrine disorders.
Exclusion criteria were adolescents < 12 years old or > 18 years old, underweight (18.5 kg/m2 BMI), overweight (25–29.9 kg/m2), or obese (BMI > 30 kg/m2) [12], with irregular menstrual cycles, known medical disorders (i.e. diabetes or hypertension), known endocrine disorders (i.e. thyroid or hyperprolactinaemia), those who received exogenous hormones within the last year, and/or refused to participate.
Regular menstrual cycles are defined as menstrual flow on a regular basis every 21–35 days.
Diabetes is defined by the American Diabetic Association as a group of metabolic disorders characterized by hyperglycaemia resulting from either deficient insulin secretion and/or insulin action, and diagnosed when the HbA1c ≥ 6.5% and fasting plasma sugar ≥ 126 mg/dl (7 mmol/l), or 2-hour plasma glucose ≥ 200 mg/dl (11.1 mmol/l) [13].
Hypertension is systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg (on 2 different days) [14].
Blood samples were collected from the studied participants to measure the serum thyroid stimulating hormone (TSH), free thyroxine (T4), prolactin (normal < 29 ng/ml) [15], glycosylated haemoglobin (HbA1C), (normal < 6.5%) [13], and 25(OH)D.
The normal TSH range is 0.4–4.0 mIU/ml, while the normal free T4 range is 0.9–2.3 ng/dl [16].
The subclinical hypothyroidism was diagnosed when the TSH was mildly elevated with normal free T4. The clinical/overt hypothyroidism was diagnosed with high TSH and low free T4 [16].
The serum 25(OH)D was measured using the spectrophotometric method because it forms a pink chloroform that can be read at 500 nm wavelength when it binds with the antimony trichloride.
A serum level of 25(OH)D > 30 ng/ml was defined as normal serum vitamin D, while the vitamin D deficiency was diagnosed at serum 25(OH)D < 20 ng/ml [17].
The studied participants were classified according to their 25(OH)D levels into 2 groups: a 25(OH)D- deficient group (study group) and a control group (normal 25(OH)D level), to detect the relationship between 25(OH)D and adolescents’ hypothyroidism.
Statistical analysis
The G Power 3.1.9.7 with 0.05 probability, 0.95% power, 0.5 sample size, and Student t-test for statistical analysis was used to calculate the sample size [18]. Student’s t-test, correlation analysis (Pearson’s correlation), and MedCalc. 20.106 [Odd ratio (OR) MedCalc. Software Ltd., Belgium] were used for statistical analysis. P < 0.05 was considered significant.
Ethical considerations
Participants were included in this research after approval (No. 10 dated 04.10.2020) from the Ethics Committee of West Kazakhstan Medical University and after informed consent following the Helsinki Declaration from the participants themselves and their guardians.
Grant funding for scientific and/or technical projects for the years 2021–2023 – Republic of Kazakhstan – Features of bone tissue metabolism and mineral density in teenage girls with primary dysmenorrhea – IRN AP09563004 – Supervisor (AA).
Results
A total of 180 adolescents between 12–18 years old were included in this cross-sectional research to detect the relationship between 25(OH)D and adolescent hypothyroidism.
A serum level of 25(OH)D > 30 ng/ml was defined as normal serum vitamin D, while vitamin D deficiency was diagnosed at serum 25(OH)D < 20 ng/ml [17]. The studied participants were classified according to their 25(OH)D levels into a 25(OH)D-deficient group (study group) and a control group [normal 25(OH)D level] to detect the relationship between 25(OH)D and adolescent hypothyroidism.
Clinical/overt hypothyroidism was diagnosed with high TSH and low free T4. Subclinical hypothyroidism was diagnosed when the TSH was mildly elevated with normal free T4 [16].
There was no statistical difference between the 25(OH)D-deficient group and controls regarding the mean weight (58.7 ±2.05 kg, vs. 58.1 ±2.8, respectively) (p = 0.99), height (157.7 ±1.52 cm vs. 157.6 ±2.4, respectively) (p = 0.99), and BMI (23.5 ±0.7 kg/m2 vs. 23.35 ±0.8, respectively) (p = 0.89) (Table 1).
Table 1
Participants’ characteristics, thyroid stimulating hormone, free thyroxine, and 25(OH)D
The 25(OH)D was statistically lower in the 25(OH)D- deficient group than in the normal controls (13.8 ±2.5 ng/ml vs. 35.6 ±2.01, respectively) (p = 0.02; 95% CI: –22.5, –21.8, –21.1). The thyroid stimulating hormone was statistically higher in the 25(OH)D-deficient group than in the normal controls (3.71 ±1.4 mIU/ml vs. 2.67 ±0.99) (p = 0.0006; 95% CI: 0.68, 1.04, 1.4), and the free T4 was statistically lower in the 25(OH)D-deficient group than in the normal controls (1.4 ±0.56 ng/ml vs. 1.5 ±0.4) (p = 0.0008, 95% CI: 0.24–0.1, 0.044) (Table 1).
The 25(OH)D-deficient group had higher odds of subclinical hypothyroidism [OR 4.89 (95% CI: 1.34–17.82), p = 0.016], and clinical/overt hypothyroidism compared to controls [OR 4.3 (95% CI: 1.37–13.5), p = 0.013] (Table 2).
Table 2
The odds of subclinical and clinical hypothyroidism in the 25(OH)D-deficient group vs. controls
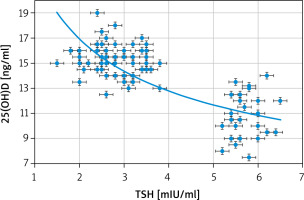
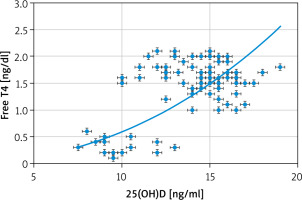
Significant negative correlations between the 25(OH)D and both clinical and subclinical hypothyroidism (r = –0.829; p < 0.00001), and between 25(OH)D and TSH (r = –0.793; p < 0.00001) were detected in this study (Fig. 1). Additionally, there was a significant positive correlation between 25(OH)D and te free T4 (r = 0.55; p < 0.00001) (Fig. 2).
Discussion
Vitamin D has a potent immunomodulatory effect and plays an important role in the pathogenesis of autoimmune diseases [19].
Serum 25(OH)D is a reliable indicator of vitamin D status. It reflects cutaneous production of vitamin D and vitamin D intake [20], and it has a long half-life [8, 9].
The serum 1,25(OH)2D level is not a reliable marker of vitamin D status because it is only decreased after severe vitamin D deficiency [9]. Additionally, it has a short half-life, and it is regulated by the PTH, calcium, and phosphate [20].
Therefore, 180 adolescents between 12–18 years old were included in this cross-sectional research, to detect the relationship between 25(OH)D and adolescent hypothyroidism. The studied participants were classified according to their 25(OH)D levels into 2 groups: a 25(OH)D-deficient group (study group) and a control group [normal 25(OH)D level].
There was no statistical difference between the 25(OH)D-deficient group (study group) and the controls regarding the mean weight (p = 0.99), height (p =0.99), and BMI (p = 0.89).
The 25(OH)D was statistically lower in the 25(OH)D-deficient group than in the normal controls (13.8 ±2.5 ng/ml vs. 35.6 ±2.01, respectively) (p = 0.02). Thyroid stimulating hormone was statistically higher in the 25(OH)D-deficient group than in the normal controls (3.71 ±1.4 mIU/ml vs. 2.67 ±0.99) (p = 0.0006), and the free T4 was statistically lower in the 25(OH)D- deficient group than in the normal controls (1.4 ±0.56 ng/ml vs. 1.5 ±0.4) (p = 0.0008). The 25(OH)D-deficient group had higher odds of subclinical hypothyroidism (OR 4.89; p = 0.016) and clinical hypothyroidism compared to controls (OR 4.3; p = 0.013).
Similarly, Chao et al. found that the TSH was significantly higher and the free T4 was significantly lower in the 25(OH)D-deficient group compared to normal controls [21].
However, Szulc et al. found that vitamin D has an immune-modulator effect and it influences AITDs, including HT, and GD [22].
Bozkurt et al. reported that patients with hypothyroidism, irrespective of whether the hypothyroidism was due to AITDs or not, had a lower vitamin D level (p < 0.05) [23].
In addition, Ke et al. observed low 25(OH)D levels in the mild and treated HT patients, while the GD patients had similar 25(OH)D levels to those of healthy controls [24].
Significant negative correlations between the 25(OH)D and both the clinical and subclinical hypothyroidism (r = –0.829; p < 0.00001), and between the 25(OH)D and TSH (r = –0.793; p < 0.00001) were detected in this study. Additionally, there was a significant positive correlation between the 25(OH)D and free T4 (r 0.55; p < 0.00001).
Kim et al. found that vitamin D insufficiency was associated with AITDs and HT, especially overt hypothyroidism [25]. Kim et al. also found that lower serum vitamin D was independently associated with high serum TSH [25].
Fang et al. confirmed a positive correlation between antithyroid antibodies and vitamin D deficiency (OR 2.428, 95% CI: 1.38–4.26) [26].
Moreover, Mackawy et al. showed that serum 25(OH)D was significantly lower in hypothyroidism compared to normal controls, with a significant association between vitamin D deficiency and hypothyroidism, and a negative correlation between vitamin D and TSH levels [27].
Recently, Appunni et al. analysed 7943 participants and found that 25.6% of those with hypothyroidism had deficient vitamin D, compared to 20.6% of healthy controls [28]. They also found that the odds of hypothyroidism were significantly higher among the vitamin D-deficient population [28].
The association between hypothyroidism and vitamin D deficiency can be explained by 2 mechanisms. First, poor intestinal absorption of vitamin D in hypothyroidism [10], and second, the VDR gene polymorphisms can predispose to AITDs [10].
Autoimmune thyroid disorders are due to the host’s immune response against the native antigens [i.e. TSH, TSH-receptors, thyroglobulin, or thyroid peroxidase (TPO)]. Anti-TPO and anti-thyroglobulin antibodies are commonly associated with HT [29]. Hashimoto’s thyroiditis is common cause of hypothyroidism in the iodine-sufficient population [25], and it is characterized by infiltration of thyroid follicles by lymphocytes [30], with subsequent thyroid follicle destruction [25].
Krysiak et al. found that 25(OH)D was inversely correlated with the thyroid antibody titre, and vitamin D intake may reduce the thyroid autoimmunity in levothyroxine-treated women with HT [30].
Bozkurt et al. found that the 25(OH)D level was statistically lower in HT participants than in normal controls (p < 0.001), and the severity of vitamin D deficiency was correlated with the duration of HT and the TPO/thyroglobulin antibody titre (p < 0.001) [23].
This study was the first cross-sectional study conducted in West Kazakhstan (Aktobe) to detect the relationship between 25(OH)D and adolescents’ hypothyroidism.
This study found that TSH was statistically higher and free T4 was statistically lower in the 25(OH)D-deficient group than in normal controls. The 25(OH)D-deficient group had higher odds of both subclinical and clinical hypothyroidism compared to controls.
Significant negative correlations between 25(OH)D and both the clinical and subclinical hypothyroidism (r = –0.829; p < 0.00001), and between 25(OH)D and TSH (r = –0.793; p < 0.00001) were detected in this study.
Failure to detect the causes of hypothyroidism in the studied participants, and the effect of vitamin D supplementation on hypothyroidism (because of the cross-sectional nature of the study) were limitations of this study. The effect of vitamin D supplementation on the hypothyroidism need to be evaluated in further studies. This study recommends vitamin D screening for individuals at high-risk of hypothyroidism.
Conclusions
Thyroid-stimulating hormone was statistically higher and free T4 was statistically lower in the 25(OH)D- deficient group than in the normal controls. The 25(OH)D- eficient group had higher odds of both subclinical and clinical hypothyroidism compared to controls. A significant negative correlation between 25(OH)D and TSH, and a significant positive correlation between 25(OH)D and the free T4 were detected in this study.