Introduction
Congenital diaphragmatic hernia (CDH) is a defect involving herniation of the abdominal organs into the pleural cavity through an incompletely closed diaphragm. This defect is also accompanied by various types of pulmonary and bronchial hypoplasia and serious anomalies in the pulmonary vascular bed and pulmonary hypertension. The cause of CDH is unclear. Clinical symptoms in neonates with CDH are closely related to the severity of pathomorphological changes in the respiratory system and the size of the diaphragmatic defect. The diagnosis of CDH is an indication for surgical treatment which involves the repositioning of the viscera into the abdominal cavity and repair of the diaphragmatic defect. Some patients have relatively stable general health after birth and can be operated on in the first days of life. In high-risk neonates (with severe hypoplasia and pulmonary hypertension), this repair procedure is postponed until the cardiopulmonary parameters are stabilized and pulmonary pressure reduced [1]. This can be achieved with intensive therapy, especially in patients with progressive respiratory failure, with the use of high-frequency mechanical ventilation, inhaled nitric oxide therapy, administration of surfactants, and pharmacological treatment of circulatory disorders. Extracorporeal membrane oxygenation (ECMO) is used in extreme cases resistant to standard treatment. There are no reports clearly indicating the optimal time to make a decision about the repair of CDH in paediatric patients on ECMO [2]. Three options of surgical repair exist for these patients [3]: early repair: < 72 hours on ECMO, late repair: > 72 hours on ECMO, repair after discontinuation of ECMO (post-decannulation).
Aim
Each operation in a neonate with CDH on ECMO is a challenge for the whole medical team involved in the procedure.
The aim of the study was to present the experience of surgeons from the Polish Mother’s Memorial Hospital Research Institute in Lodz (ICZMP), Department of Paediatric Surgery, Urology and Transplantology, Department of Intensive Care and Congenital Defects of Infants and Neonates, regarding congenital diaphragmatic hernia repair in neonates on extracorporeal membrane oxygenation.
Material and methods
In 2014–2018, 126 neonates with congenital diaphragmatic hernia were surgically treated at ICZMP. Of these, 22 required treatment with ECMO. Five of them died despite being on ECMO before a decision about surgery was made. CDH was surgically repaired in another 17 neonates, and of these 7 were on ECMO.
A retrospective analysis of medical records for 7 patients with congenital diaphragmatic hernia repair on ECMO was performed with a focus on: data relating to the course of pregnancy and neonate status at birth (gestational age, body weight, Apgar score, coexisting congenital defects); time of ECMO initiation; time of CDH repair; size of diaphragmatic defect and type of herniated organs according to the CDH SG’s staging system [4]; type of repair and intraoperative conditions/difficulties; postoperative complications; time of ECMO discontinuation; patient follow-up data.
Results
The analysed group comprised 4 boys and 3 girls. The gestational age was in the range of 35–41 hbd (mean 37.4 hbd), and the birth weight in the range of 2350–3300 γ (mean: 3014 g). All neonates had left-sided congenital diaphragmatic hernia. Fetal tracheal occlusion (Fetendo-PLUG, fetal endoscopic tracheal occlusion) was performed in 2 patients. The Apgar score assessed in neonates at 1, 5 and 10 minutes was in the range of 2 to 8 points. All neonates in this period had symptoms of central cyanosis manifested mainly by bluish colour of skin and lips. Characteristics of the study group are presented in Table I.
Table I
Data on the course of pregnancy and status of the patient at birth
| Sex | HBD | Birth weight [g] | Apgar | Fetendo-Plug |
|---|---|---|---|---|
| M | 35 | 3300 | 6/6/7 | + |
| M | 41 | 3150 | 6/6/4 | |
| M | 37 | 3300 | 6/5/5 | |
| M | 38 | 3100 | 7/5/5 | + |
| F | 38 | 2800 | 4/6/7 | |
| F | 37 | 3100 | 2/5/5 | |
| F | 36 | 2350 | 8/5/7 |
All patients were intubated and mechanically ventilated promptly in the delivery room, but in further hours of life, despite ventilation and modification, presented with more and more severe symptoms of respiratory failure resulting from pulmonary hypoplasia and pulmonary hypertension (confirmed by echocardiography). Two neonates had coexisting defects, i.e. tetralogy of Fallot (n = 1) and kidney defect (n = 1).
Due to the extremely severe condition of the neonate and ineffective treatment with mechanical ventilation and inhaled nitric oxide therapy, the decision was made to initiate ECMO in 5 newborns on the first day of life, and in 2 other patients on the second day of life.
In 3 patients CDH repair with the repositioning of the viscera from the pleural cavity was performed 24 hours after the initiation of ECMO (early repair < 72 hours). In another 4 patients the surgery was done on days 4, 5, 7 or 11 of life (late repair > 72 hours) (Table II).
Table II
Time of ECMO initiation and repair of congenital diaphragmatic hernia
Intraoperatively, a large hole in the left diaphragmatic dome with partial agenesis of the rear portion of the diaphragm (type C) was detected in the majority of patients (n = 5). One patient had a larger defect (type B), and 1 patient had left diaphragmatic agenesis (type D).
Intraoperatively, herniation of the stomach, small intestine, large intestine and spleen into the chest was detected in all neonates. In 5 neonates we also found the liver (mainly left lobe) herniated into the pleural cavity. In 2 patients the whole liver was not displaced and located in the abdominal cavity. In 6 neonates the visceral organs were deherniated into the abdominal cavity and the left side of the diaphragm was repaired with a patch of artificial material Gore-Tex (Gore-Tex – Soft Tissue Patch, Flagstaff, Arizona, USA). Only in 1 patient with type B defect was it possible to repair the hernia without the need to use synthetic material. In 1 patient a Gore-Tex patch had to be used to close the abdominal cavity.
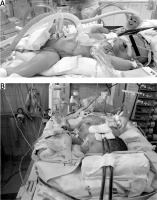
The following surgical problems were encountered intraoperatively: positioning of the patient (Figures 1 A, B). forced by the cannulae inserted in the carotid vessels during ECMO (n = 7); significant generalized oedema (n = 7) observed in all patients on ECMO; significant capillary bleeding from the surgical wound observed in 5 neonates (haemostatic material TachoSil from Takeda, Austria, was used); difficulties with closing the abdominal cavity (n = 4) and the need for the temporary use of a Gore-Tex patch as a skin substitute (n = 1).
The most common postoperative complications included: bleeding into the left pleural cavity (n = 3), mainly in the first 24 hours after the surgery, and in 1 patient 14 days after the surgery (treated by drainage of the chest). One patient required reoperation – a thoracotomy revealed multiple superficial haemorrhagic sites that disappeared after a seton placement); bleeding from the surgical wound (n = 1) (treated by placement of an additional suture on the skin); bleeding from the bronchial tree (n = 1) (treated by reducing the infusion of heparin); bleeding from the urinary tract (n = 1) (treated by reducing the infusion of heparin); bleeding from the gastrointestinal tract (stomach) on day 1 after surgery and relapse of bleeding on day 5 (n = 1) (treated by reducing the infusion of heparin); bleeding into the supratentorial structures (n = 1) on day 8 after ECMO discontinuation.
There were also perioperative problems directly related to the initiation of ECMO, i.e. bleeding at the cannula insertion site (n = 1), displacement of the cannula and the need for its repositioning on the first day after surgery (n = 1), and redness of the skin and subcutaneous tissue of the neck in sites after decannulation (n = 1). One patient had a relapse of a diaphragmatic hernia 3 weeks after the surgery. The patient was disconnected from ECMO on day 9 of life and then mechanically ventilated. On reoperation, a partial disintegration of the patch in the lateral angle was detected. A larger patch was used to close the wound. The patient was discharged on day 76 of life.
Another patient in whom ECMO was discontinued on day 14 of life presented with symptoms of acute gastrointestinal obstruction in week 3 of life and required urgent reoperation, which involved visual examination of the abdominal cavity, the release of intestinal adhesions, and resection of a portion of the jejunum due to the necrosis of the wall. The patient was discharged in month 7 of life.
There were five fatal cases in the study group. Three patients died while on ECMO, and another two on days 1 and 10 after decannulation. In 4 cases the cause of death was multiple organ failure. One infant died 30 minutes following decannulation as a result of massive bleeding from a ruptured aneurysm that formed in the wall of the cannulated vessel. Of all 7 patients operated on while on ECMO 2 survived and are still receiving ambulatory care. ECMO was discontinued in these patients on day 9 or 14.
Discussion
Despite the tremendous progress that has been achieved in the diagnosis and treatment of neonates with CDH, mortality in this group of patients is still high. It is associated primarily with the immaturity of the lungs and the abnormal structure of the pulmonary vasculature, leading to the development of pulmonary hypertension. These factors largely determine the survival of patients with CDH.
For many years research has continued to identify factors with predictive value in neonates suffering from CDH. Most treatment models based on national databases rely on factors such as birth weight, Apgar score, usually at 5 minutes after birth, PaO2 and PCO2 values, the presence of pulmonary hypertension, and the coexistence of other birth defects [5].
Table I presents characteristics of patients from the analysed group. Data show that only 1 patient was born prematurely (35 hbd), but his birth weight was 3300 g. This patient was treated with fetal endoscopic tracheal occlusion. This therapy was used in 2 patients from the study group. The second infant was born at term with a birth weight of 3100 g. The mean birth weight in the group of analysed infants was 3014 g, and the mean gestational age was 37.4 hbd. Reportedly, these parameters indicate a good prognosis. Prematurity is a recognized factor affecting survival and prognosis, especially in newborns with a serious disease or birth defect, including CDH. There are some reports with recommendations on the delivery of children with diagnosed CDH after 39 weeks of gestation [6]. On the other hand, Stevens et al. [7] presented findings indicating that early-term birth (37–38 hbd) through caesarean section was associated with lower risk related to the use of ECMO [7]. There are also some reports suggesting that fetal tracheal occlusion increases the chances of survival, especially in neonates with CDH who would not survive without this procedure [6, 8]. However, there are still no data from randomized trials legitimizing the use of this procedure. A high risk of preterm delivery associated with fetal tracheal occlusion was also reported [9].
The incidence of coexisting defects in the analysed children was also relatively low. Two infants had coexisting defects: tetralogy of Fallot (n = 1) and kidney defects (n = 1).
The size of the diaphragmatic defect is a prognostic factor in patients with CDH [10–12]. A positive correlation between the size of the defect, the survival rate, and the risk related to ECMO has been reported. Large defects, usually requiring the use of synthetic material to repair the diaphragm, also have a negative effect on the survival of a newborn with CDH [10–12]. In the group analysed in our study we were able to repair the diaphragm without the need for grafting artificial material only in 1 patient. In other patients the diaphragmatic defect was closed using a Gore-Tex patch.
Precise recommendations for the care of neonates with CDH have been set out in the Neonatal Resuscitation Program (NRP) by the American Academy of Pediatrics [13]. One of these recommendations is the prompt intubation of patients with congenital diaphragmatic hernia in the delivery room [14]. At our institute all neonates are intubated in the delivery room. This principle was also applied in all patients in the study group.
Pulmonary hypoplasia in neonates with CDH results from the inhibited branching of the bronchial tree, reduced number of alveoli, and hypoplasia of the pulmonary vascular bed due to underdevelopment of the capillary vasculature and reduced diameter of arterioles caused by significant hypertrophy of the muscular layer. These anomalies are observed not only in the lung on the side of the defect, but also, although to a lesser extent, on the contralateral side. Anomalies reduce the surface of gas exchange in the lungs, and promote the onset and development of respiratory and circulatory failure in neonates with CDH [15]. Reportedly, these factors have a more significant impact on the status and survival of the patient than the volume of abdominal organs herniated into the chest.
Extracorporeal blood oxygenation (ECMO) is indicated in neonates who, despite intensive treatment (mechanical ventilation, inhaled nitric oxide therapy, administration of surfactants and catecholamines, normalization of water and electrolyte imbalance, etc.), still show no clinical improvement [16, 17]. For technical reasons, this procedure can only be used in neonates with a birth weight above 2000 γ and gestational age over 35 weeks. ECMO is a technique that involves the temporary (up to several days) use of an extracorporeal device performing the function of the patient’s heart and lungs. Because of numerous severe complications (intracranial haemorrhage, ischaemic stroke, bleeding from the gastrointestinal and respiratory tracts, seizures, systemic infections), a strictly defined group of newborns with CDH with acute respiratory and/or circulatory failure resistant to conventional therapy are qualified for this procedure [16, 17]. Table III presents updated recommendations of the CDH EURO Consortium on the use of ECMO in neonates with CDH [18]. Based on these recommendations, 5 neonates from our study group were qualified and put on ECMO on day 1 of life, and other patients on the second day of life.
Table III
Updated recommendations on the use of ECMO in neonates with CDH [18]
There are some controversies about the time of surgery in patients on ECMO. The most beneficial procedure would be to carry out surgery before qualifying a neonate for ECMO, and if ECMO is required, then the surgical procedure should be performed on the first days on ECMO. According to literature data, the time of support on ECMO is shorter in these patients, and the risk of complications is reduced, which results in better patient survival [17]. However, some reports indicate an increased risk of intraoperative bleeding during procedures performed up to 72 hours after the initiation of ECMO [19]. The optimal timing of surgery in these patients with a severe condition is extremely difficult, has to be personalized, and depends on the skills and experience of the medical team in charge of the patient.
In our study, early surgical repair of CDH, i.e. within 72 hours on ECMO, was done in 3 neonates. Other patients were operated on at a later date, i.e. on days 4, 5, 7 or 11 after the initiation of ECMO. This difference in timing of the surgery resulted from the general status of patients.
There are no reports regarding intraoperative complications during the surgical repair of CDH in neonates on ECMO. Our experience, although narrow, shows that the most significant problems include: forced position of the patient, significant oedema of tissues, capillary bleeding from the surgical wound, difficulties with closing the abdominal cavity.
The repair of left CDH performed using the classical technique with abdominal access is usually done in a patient with a slightly elevated epigastrium. The position of the patient on ECMO with the cannulation of carotid vessels poses considerable limitations. Any preoperative and intraoperative manipulation of the patient’s position may cause the dislocation of cannulas, which can disturb blood flow, and cause bleeding in the cannulated site and damage to vessels in extreme cases. Therefore, when repair is done with abdominal access, especially in the smallest patients placed on the back with the head and neck immobilized because of the cannulation of carotid vessels, there is a limited possibility of even a slight bending or elevation of the body, which often makes surgical access to the left hypochondrium difficult and requires extensive experience from the operator. Systemic oedema and capillary bleeding observed in all patients create additional difficulties with manipulation within the operating field in neonates on ECMO. Oedema is primarily due to the patient’s severe condition associated with acute cardiorespiratory failure. Swollen tissues are fragile, which is particularly problematic when closing the abdominal cavity. Low capacity of the abdominal cavity caused by herniation of the viscera during the prenatal and postnatal period may be another obstacle to the primary closure of the abdominal wall, particularly in patients with CDH types C and D. In 4 patients from our study group generalized oedema and fragility of tissues created additional difficulties in closing individual layers of the abdominal wall, and in 1 patient the operator had to graft a temporary Gore-Tex patch to close the peritoneum and muscles of the abdominal wall.
Intensive intraoperative bleeding, especially from capillaries, was observed in 5 neonates from the study group. This bleeding was mainly associated with heparin administration during ECMO therapy. Therefore, intraoperative haemostasis should be maintained in patients during surgical repair, vessels should be covered to control bleeding, especially from capillaries, and haemostatic materials can be used for that purpose. Because of bleeding, mainly from capillaries, a haemostatic sponge (TachoSil) had to be used during the surgery in 5 patients. Bleeding is the most common complication associated with ECMO therapy. It is reported in about 30% of patients on ECMO, and usually occurs in the cannulated site (7.6%), central nervous system (7.4%), postoperative wound (6.3%), and gastrointestinal tract (1.6%) [20]. ECMO is associated with the need for long-term heparinization. Patients need frequent administration of blood substitutes, transfusion of platelets and antithrombin III, and frequent monitoring of coagulation parameters and blood count.
Most postoperative complications in the study group were associated with bleeding. In 3 cases it was bleeding into the pleural cavity observed in the first days after surgery, and in 1 patient on day 14 after surgery.
The survival rates in patients who had a repair of a congenital diaphragmatic hernia on ECMO reported by Kays were estimated at 73% for early repair, 50% for late repair and 64% for post-decannulation repair [21]. Moreover, Kays demonstrated a lower incidence of complications (bleeding) in neonates who had early repair of CDH. Similar findings were reported by Dassingner et al., who estimated the survival at 71% in patients with CDH repaired on average 55 h after ECMO initiation [22]. Golden et al. estimated the survival of patients with CDH on ECMO at 36% for late repair and 85% for post-decannulation repair [23]. Because of the diversity of data, the small number of studies carried out in this area, and the heterogeneity of patient groups, it is impossible to clearly determine the optimal time to perform the repair of CDH in children with recommendations for ECMO.
Conclusions
The choice of optimal time for the surgical repair of CDH in neonates on ECMO is difficult and requires an individualised approach for each patient. Surgeons have to consider difficulties resulting from the forced position of the patient connected to the ECMO machine, intensive bleeding related to the treatment, generalized oedema, and problems with the closure of the surgical wound and skin. Internal bleeding may also be a serious postoperative and life-threatening complication. Our experience with the surgical treatment of paediatric patients on ECMO is still narrow, and each new case will be regarded as another challenge.