Introduction
Pulmonary large cell neuroendocrine carcinoma (LCNEC), of which the incidence is estimated to be approximately 3% among all primary lung tumors, is one of the rare malignant neoplasms of the lung [1]. The clinical features and therefore management of LCNEC are more similar to small cell lung carcinoma. However, LCNEC have been classified as a neuroendocrine carcinoma, a subgroup of non-small cell lung carcinoma, by the World Health Organization since 2015 as they have neuroendocrine cell features under the light microscope and also in immunohistochemical staining [2]. A standard management model for LCNEC has not yet been established, as they are rare and related studies are generally retrospective with small populations. However, their prognosis can be as poor as small cell carcinoma, and the poor prognostic factors and treatment modalities are still uncertain.
Aim
In this study, we investigated the clinical features and survival rates of patients with LCNEC in order to define the factors related to the prognosis and we found that among the clinical features we investigated, tumor size and TNM stage were associated with low survival rates.
Material and methods
There were 46 patients diagnosed with large cell neuroendocrine carcinoma (LCNEC) between January 2010 and December 2021. Four patients whose surgical staging could not be performed were excluded from the study. Therefore, we analyzed the data of 42 patients in this study.
We obtained the data about patients’ age, gender, smoking history, symptoms, tumor size, tumor location, pathological type (pure versus mixed), tumor size-lymph node-metastasis (TNM) stage, treatments (surgery, chemotherapy, radiotherapy), surgical modality (sublobar resection, lobectomy, pneumonectomy), length of hospital stay, postoperative complications, disease-free survival and total survival, from the hospital electronic files of the patients. All patients were re-classified according to the AJCC/UICC 8th Edition TNM staging system [3].
The relationship between age, gender, pathological type (pure versus mixed), tumor size, TNM stage, treatment modalities, postoperative surgical complications and survival were analyzed.
Ethical issues
This study was conducted in accordance with the Helsinki Declaration Rules after receiving approval from the Clinical Research Ethics Committee of Akdeniz University Faculty of Medicine. (Date: 09/03/2022 Number:70904504/39).
Statistical analysis
The collected data were entered into the database in the program IBM SPSS 23.00 and all statistical analyses were performed in the same program. Continuous variables were expressed as arithmetic mean, standard deviation, minimum and maximum values, and categorical variables were expressed as frequency and percentage. Univariate and multivariate Cox regression analyses of potential factors affecting patients’ outcome were performed. Non-multicollinearity of the grouped covariates was checked. Significance level in the univariate model for inclusion in the multivariate model was set at 0.2. The Kaplan-Meier method was used for survival curves.
Results
Patients’ characteristics
Of the 42 patients whose pathological diagnosis was reported as LCNEC, 2 (4.76%) were female and forty (95.24%) were male, and the mean age was 64.26 ±8.62 (41–79 years). Thirty-four (80.95%) patients were current/ex-smokers. The tumor was detected incidentally in 14 (33.33%) of the patients. The most common symptom was cough, which was present in 18 (42.85%) patients.
Tumor characteristics
The median tumor size was 4.1 (1.1–8.2) cm and 33 (78.6%) of them were localized in the periphery of the lung. 34 (80.95%) of the LCNEC were pure type and 8 (19.05%) were mixed type. Six (14.28%) of the patients were in T4N1M0, 6 (14.28%) of them were in T2aN0M0 and 5 (11.90%) were in T1bN0M0. Twelve (28.57%) of the patients were in stage I, 14 (33.3%) of them were in stage II, 15 (35.71%) were in stage III and only 1 (2.38%) patient was in stage IV.
The patients and tumor characteristics and the distribution of TNM staging which was determined after mediastinal lymph node sampling are given in Table I.
Table I
Demographic characteristics of patients, surgical characteristics and postoperative-complication rate
While 13 (30.95%) patients were operated on only for diagnostic purposes due to the advanced stage, curative surgical interventions were performed in all of the early-stage patients (n = 29). The right upper lobectomy was the most common surgical modality (n = 10). Fifteen (35.71%) had sublobar resection (wedge resection (n = 13) + segmentectomy (n = 2)), 24 (57.14%) had lobectomy and 3 (7.14%) had pneumonectomy.
There were postoperative complications in 9 (21.42%) patients (5 patients with right upper lobectomy and 3 patients with wedge resection and 1 patient with lobectomy). The most common postoperative complications were pneumonia (n = 4) and prolonged air leakage (n = 4). Empyema developed in 2 of the patients with prolonged air leakage. None of the patients who developed postoperative complications required a second surgical intervention. Massive embolism and disseminated intravascular coagulopathy developed in 1 patient postoperatively, and this patient died on the 5th postoperative day. The hospital stay of the patients was determined as 9.38 ±8.82 days. When the patient who died on the 5th postoperative day and the patient who was hospitalized for 59 days due to the development of empyema were excluded, the hospitalization period of the patients was determined as 8.25 ±4.11 days.
Postoperative treatment, follow-up and prognosis
Thirty-five patients had adjuvant platinum-based chemotherapy, or radiotherapy or both. The remaining 7 (16.66%) patients did not receive postoperative adjuvant therapy, including 5 stage I patients, 1 stage II patients, 1 stage III patient. During follow-up, of those who received postoperative adjuvant therapy, 24 survived and 11 died while of those who did not receive postoperative adjuvant therapy, 5 survived and 2 died.
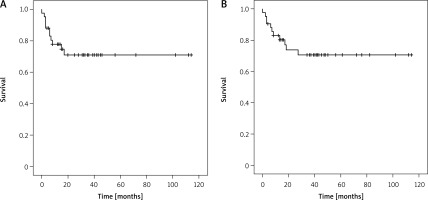
For all 42 patients, the mean disease-free survival time was 28.14 ±28.49 months and the overall mean survival time was 34.86 ±30.11 months. 1-year, 3-year and 5-year survival rates of the patients were 73.80%, 47.61% and 19.04%, respectively. Survival and disease-free survival curves of the patients were determined by the Kaplan-Meier method and are shown in Figure 1.
Risk factors associated with survival
Univariate log-rank analysis suggested that tumor size and nodal staging were factors associated with overall survival. Tumor size was significantly higher in non-survivors compared to survivors. Non-survivors had a significantly higher tumor size and nodal stage than the survivors (Table II). There were no significant differences in terms of age, gender, pathological type, treatment modality and the presence of postoperative complications between survivors and non-survivors.
Table II
Univariate log-rank analysis and multivariate analysis of prognostic factors
The Cox multivariate model results suggested that surgical treatment modality (HR = 2.156, 95% CI: 0.655–21.942, p = 0.139), T stage (HR = 8.956, 95% CI: 1.521–11.034, p = 0.005), N stage (HR = 5.984, 95% CI: 1.127–7.982, p = 0.028), M stage (HR = 2.854, 95% CI: 0.489–17.467, p = 0.239) and TNM stage (HR = 11.159, 95% CI: 2.783–36.182, p = 0.001) were independent risk factors for OS in LCNEC patients (Table II).
Discussion
LCNEC, a subgroup of malignant neoplasms quite different from the other primary lung tumors in terms of morphological and clinical features, may have a poor prognosis. However, the factors associated with prognosis of LCNEC are uncertain. Here, in this study, we investigated the clinical features and survival rates of patients with LCNEC in order to define the factors related to the prognosis and we found that among the clinical features we investigated, tumor size and TNM stage were associated with low survival rates.
LCNEC of the lung are rare tumors that make up 1–3% of all primary lung cancers. Therefore, there are many uncertainties about this group of lung tumors. According to data from several retrospective studies with small populations, LCNEC are more common in elderly male patients with a history of heavy smoking [4, 5]. The findings of our study were consistent with these data, and almost all of the patients in our study were middle-aged/elderly male patients with a smoking history. Age and the gender were not associated with survival in our study.
LCNEC can be solitary or multiple, but they are certainly more frequent in the periphery of the lung [5–7]. With features such as peripheral localization and lobulated, well-demarcated margins, they can mimic a peripheral small cell lung carcinoma or a poorly differentiated adenocarcinoma and for the definitive diagnosis histopathological evaluation is required [8, 9]. Typical histopathological features are non-small cell cytological features (large cell size, low nuclear/cytoplasmic ratio, frequent and often prominent nucleoli) and neuroendocrine morphology (organoid nesting, trabecular, rosette-like and palisading patterns). For the differentiation of neuroendocrine cells, immunohistochemical staining for chromogranin A, synaptophysin, and neural cell adhesion molecule are used. Otherwise, it can be difficult to differentiate it from other subtypes of large cell carcinoma, atypical carcinoma and small cell lung carcinoma. It can still be challenging for the pathologist to diagnose, especially when small samples were obtained [2, 5]. For this reason, in the current study, all the samples were surgically obtained and furthermore we performed surgical lymph node staging in all patients. Moreover, most of the LCNEC in our study were also peripherally localized. However, the localization of the tumor, either peripheral or central, was not associated with survival.
Patients with LCNEC have a poor prognosis. LCNEC survival curves are similar to small cell lung cancer (SCLC), with high recurrence rates and short overall survival rates, even in patients with early-stage disease [4, 10–13]. In a previous study, 5-year survival rate and 5-year disease-free survival rate were found to be 35% and 27%, respectively, for all stages, and most of the relapses occurred in the first 2 years of follow-up [5, 14]. In a retrospective cohort study of 57 patients previously performed to analyze clinical and immunohistochemical determinants of postoperative survival, advanced stage and advanced nodal involvement were found to be associated with worse prognosis [15]. TNM stage seems to be important when deciding the treatment modality [16, 17]. Nodal staging was reported to be associated with survival in some studies. In a retrospective cohort of 57 patients with LCNEC, advanced nodal involvement was associated with a poor prognosis [15]. Zacharias et al. suggested that the systematic mediastinal nodal dissection improved the outcomes in LCNEC [18]. The correct nodal staging was associated with better survival. This finding indicates the importance of nodal staging in predicting survival in LCNEC. Tumor size may also have a guiding effect on the treatment modality. In a cohort of 1770 patients with LCNEC, the addition of adjuvant chemotherapy in patients with tumor size > 3 cm was associated with better overall survival [19]. Moreover, the addition of adjuvant chemotherapy to those < 2 cm did not provide an additional advantage. These findings also indicated the importance of tumor size in predicting the survival in LCNEC. In our study, we also found that both the nodal staging and tumor size were associated with survival.
This study also has some limitations. It is a retrospective single center study. Our population is small in number and there is no control group. Additionally, the postoperative follow-up period may not be sufficient to evaluate recurrence. There is a need for more comprehensive studies investigating the factors associated with the survival of patients with LCNEC.