Summary
The article addresses the rising incidence of minor ischemic stroke (MIS) and its associated cognitive impairments, particularly among younger populations, emphasizing the need for effective prevention strategies. The study investigates risk factors for cerebral artery stenosis (CAS) in MIS patients classified as large atherosclerosis (LAA) according to the TOAST criteria, while also exploring the relationship between cognitive function and plasma lipoprotein-associated phospholipase A2 levels. A retrospective analysis of 789 MIS patients admitted between January 2015 and December 2023 was conducted. Among these, 494 (62.61%) patients were identified with LAA, and 377 (76.32%) showed LAA-type vascular stenosis. Key risk factors for CAS included hypertension, diabetes, prior ischemic stroke, elevated fasting blood glucose, and increased plasma lipoprotein-associated phospholipase A2 levels. Multivariate analysis confirmed hypertension and elevated plasma lipoprotein-associated phospholipase A2 as independent risk factors. The predictive capability of plasma lipoprotein-associated phospholipase A2 for CAS was supported by an area under the ROC curve of 0.700. Notably, a negative correlation was observed between plasma lipoprotein-associated phospholipase A2 levels and cognitive function, as measured by the Mini-Mental State Examination (MMSE). In conclusion, LAA is a major contributor to MIS and is associated with a high prevalence of vascular stenosis. Hypertension and elevated plasma lipoprotein-associated phospholipase A2 levels are significant independent risk factors for CAS, with the latter also linked to cognitive decline.
Introduction
Minor ischemic stroke (MIS) refers to a type of ischemic stroke characterized by relatively mild clinical symptoms, long duration, and high risk of recurrence, which is different from transient ischemic attack. Elucidating the etiology of MIS is a crucial step in determining patient prognosis, guiding treatment, and selecting secondary prevention plans [1–3]. The most widely used etiology classification system in the world is the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification [4]. According to a series of studies on this classification, large artery atherosclerosis (LAA) accounts for a high proportion of stroke cases [5–7]. At present, there are no studies on the risk factors for cerebral artery stenosis (CAS) in LAA-type MIS patients.
Aim
This study aimed to comprehensively analyze the risk factors for CAS in LAA-type MIS patients to facilitate timely secondary prevention and reduce stroke recurrence.
Material and methods
Research subjects
A total of 789 acute MIS patients, 557 males and 232 females, aged 62.43 ±11.55 years, were selected from the Department of Neurology at the Third People’s Hospital of Hefei City and the Department of Neurology at the First Affiliated Hospital of Anhui Medical University in the period from January 2015 to December 2023. The National Institutes of Health Stroke Scale (NIHSS) score was 0 for 92 patients, 1 for 109 patients, 2 for 172 patients, 3 for 131 patients, 4 for 173 patients, and 5 for 112 patients, with an average score of 2.66 ±1.58 points. There were 494 cases of LAA-MS, including 328 cases of hypertension, 122 cases of diabetes, 212 cases of hyperlipidemia, 12 cases of atrial fibrillation, 106 cases of smoking, and 95 cases of ischemic stroke.
The diagnosis of MIS met the diagnostic criteria in the Guidelines for the Diagnosis and Treatment of High-Risk Non-Disabling Ischemic Cerebral Artery Events developed by the Guidelines Writing Group of the Chinese Stroke Society in 2016: NIHSS ≤ 5 points [8]. The exclusion criteria were as follows: 1) hemorrhagic cerebrovascular disease; 2) progressive stroke; 3) severe systemic diseases such as heart, lung, liver, or kidney; and 4) allergy to contrast agents, which is not suitable for head and neck computed tomography angiography (CTA) examination.
Investigation of vascular risk factors
Related vascular risk factors, including sex, age, history of hypertension, history of diabetes, hyperlipidemia, history of atrial fibrillation, history of ischemic stroke, and history of smoking, were recorded; systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured; and fasting blood sugar (BS), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C), high-density lipoprotein cholesterol (HDL), uric acid (UA), C-reactive protein protein (CRP), homocysteine (HCY), and lipoprotein-associated phospholipase A2 (Lp-PLA2) levels were measured. Additionally, all minor ischemic stroke patients were tested for cognitive function (MMSE).
Criteria for the diagnosis of intracranial and extracranial artery stenosis
After admission, patients who underwent MIS of the LAA type underwent head and neck CTA and head magnetic resonance angiography (MRA). According to the CTA and MRA results, patients were divided into two groups: those with and without cerebral artery stenosis. The intracranial cranial arteries included the intracranial C6-7 segment of the internal carotid artery, the A1 segment of the anterior cerebral artery, the M1 segment of the middle cerebral artery, the P1 segment of the posterior cerebral artery, the intracranial V4 segment of the vertebral artery, and the basilar artery. The extracranial arteries include the extracranial C1-5 segments of the internal carotid artery and the extracranial V1-3 segments of the vertebral artery. The vascular stenosis rate was calculated according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET). The vascular stenosis rate = (normal vessel diameter at the stenosis distal end – narrowest diameter of the stenosis segment)/shortest normal diameter at the stenosis distal end × 100%. A vascular stenosis rate ≤ 49% indicated mild stenosis, 50–69% indicated moderate stenosis, and 70–99% indicated severe stenosis. 0 indicates no stenosis, and 100% indicates complete occlusion. If there were two or more arterial stenoses during the statistical analysis, the highest stenosis rate was taken [9].
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software. Quantitative data are expressed as mean ± standard deviation, and count data are expressed as percentages. Binary logistic regression models were used to perform univariate and multivariate analyses of variables to screen out independent risk factors for CAS in patients with LAA-type MIS. SPSS 17.0 software was used to draw the ROC curve of lipoprotein-associated phospholipase A2 to predict cerebral artery stenosis in patients with atherosclerotic MIS. P < 0.05 was considered to indicate statistical significance.
Results
Results of different TOAST classifications and CAS in MIS patients
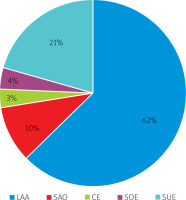
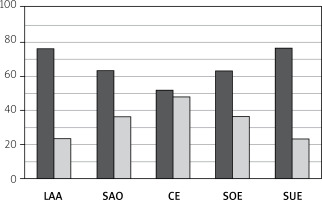
Among the 789 patients who underwent MIS, 494 (62.61%) had LAA, 77 (9.76%) had small artery occlusive disease (SAO), 25 (3.17%) had cardiogenic embolism (CE), 30 (3.80%) had stroke of other determined etiology (SOE), and 163 (20.66%) had stroke of undetermined etiology (SUE). Among them, 377 (76.32%) had LAA-type stenosis, 49 (63.63%) had SAO-type stenosis, 13 (52%) had CE-type stenosis, 19 (63.33%) had SOE-type stenosis, and 125 (76.69%) had SUE-type stenosis. LAA accounted for the highest proportion of MIS cases, and its number was far greater than that of the other types. Moreover, the proportions of patients with LAA- and SOE-type stenoses were greater. See Figures 1 and 2 for details.
Univariate logistic regression analysis of vascular risk factors in the CAS-free group and CAS group
There was no statistically significant difference in sex, age, hyperlipidemia status, history of atrial fibrillation, smoking history, SBP, DBP, TG, TC, LDL-C, VLDL-C, HDL-C, UA, CRP, or HCY levels among the groups (p > 0.05). Compared with those in the non-CAS group, history of hypertension, diabetes, ischemic stroke, fasting BS levels, and lipoprotein-associated phospholipase A2 levels in the CAS group were identified as risk factors for CAS (p < 0.05) (Table I).
Table 1
Single factor logistic regression analysis
[i] SBP – systolic blood pressure, DBP – diastolic blood pressure, BS – blood sugar, TG – triglyceride, TC – total cholesterol, LDL-C – low-density lipoprotein cholesterol, VLDL-C – very low-density lipoprotein cholesterol, HDL-C – high-density lipoprotein cholesterol, UA – uric acid, CRP – C-reactive protein, HCY – homocysteinemia, Lp-PLA2 – lipoprotein-associated phospholipase A2.
Multivariate logistic regression analysis of vascular risk factors
A history of hypertension was more common and lipoprotein-related phospholipase A2 levels were significantly greater in the CAS group (OR = 2.046, 95% CI: 1.285–3.258, p = 0.003; OR = 1.059, 95% CI: 1.041–1.077, p < 0.001) (Table II).
Table II
Multivariate logistic regression analysis
| Factor | OR | 95% CI | P-value |
|---|---|---|---|
| Hypertension | 2.046 | 1.2852–3.258 | 0.003 |
| Diabetes | 0.997 | 0.489–2.031 | 0.99 |
| History of ischemic stroke | 1.727 | 0.906–3.291 | 0.09 |
| BS | 1.135 | 0.992–1.299 | 0.06 |
| Lp-PLA2 | 1.059 | 1.041–1.077 | < 0.001 |
Lipoprotein-associated phospholipase A2 predicting ROC curve of MIS cerebral artery stenosis
The ROC curve of lipoprotein-associated phospholipase A2 in predicting cerebral artery stenosis in patients with atherosclerotic MIS is presented in the figure below (Figure 3). The results show that:
Figure 3
Lipoprotein-associated phospholipase A2 predicting ROC curve of MIS cerebral artery stenosis
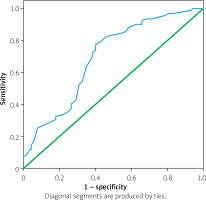
The ROC curve parameters of lipoprotein-associated phospholipase A2 in predicting cerebral artery stenosis in patients with atherosclerotic MIS were: the area under the ROC curve was 0.700, the standard error was 0.030, the 95% CI confidence interval was 0.636–0.753, and p < 0.0001. The sensitivity was 0.827, and the 1-specificity was 0.470. Therefore, by drawing the ROC curve, the optimal cutoff threshold of lipoprotein-associated phospholipase A2 in predicting cerebral artery stenosis in MIS patients was found to be 69 ng/ml.
The correlation analysis between lipoprotein-associated phospholipase A2 and MMSE
The correlation analysis between lipoprotein-associated phospholipase A2 and MMSE showed a correlation coefficient of r = –0.218, p < 0.001. The results indicate a negative correlation between lipoprotein-associated phospholipase A2 and MMSE, meaning that as lipoprotein-associated phospholipase A2 increases, MMSE decreases and cognitive impairment becomes more severe in patients.
Discussion
Minor ischemic stroke is a complex disease that might be caused by multiple underlying causes. The 90-day recurrence rate of minor ischemic stroke patients is approximately 12–20%, similar to that of transient ischemic attack patients [10, 11]. Accurate classification of stroke subtypes is crucial for accurate diagnosis, timely treatment, and prognostic evaluation. In 1993, to improve the standardization of subtype allocation in randomized controlled trials, Adams and colleagues conducted the ORG 10172 trial and proposed the TOAST criteria for the classification of ischemic stroke based on assumed etiological mechanisms. Ischemic stroke can be divided into five groups: LAA, SAO, CE, other identified causes and undetermined causes [12].
A retrospective study from Taiwan reported that the most common subtype of ischemic stroke was small artery occlusion disease (37.7%), followed by large artery atherosclerosis (27.7%) [13]. In Jordan (36%), Japan (54.1%), and Kuwait (69.8%), small artery occlusion disease was also the most common subtype of stroke. In addition, the latest research conducted by Zafar et al. revealed that small artery occlusion disease was the most common etiological subtype of ischemic stroke (32.1%), followed by cardiac embolism (21.9%) and major atherosclerosis (14.6%) [14]. However, the results of this study showed that among 789 MIS patients, LAA accounted for 494 (62.61%) cases, SAO accounted for 77 (9.76%) cases, CE accounted for 25 (3.17%) cases, other causes (SOE) accounted for 30 (3.80%) cases, and unknown causes (SUE) accounted for 163 (20.66%) cases. These findings were similar to the results of Harris et al. [12]. The proportion of patients with MIS caused by LAA in the Chinese mainland may be different from that in Taiwan or other ethnic countries, and the proportion of patients with MIS caused by LAA was much greater than that of patients with MIS caused by SAO. This suggested that atherosclerosis could play an important role in the pathogenesis of MIS in mainland China, which is different from common acute ischemic stroke diseases.
Hypertension alters the cerebral vascular system through the deposition of fibrin in blood vessels and hypertrophy of smooth muscles, leading to LAA, ultimately resulting in the narrowing or even occlusion of blood vessels in the affected area and secondary cerebral ischemia [15]. Kim [16] reported that hypertension was significantly associated with intracranial arterial stenosis. This study revealed that hypertension was an independent risk factor for cerebral artery stenosis, so blood pressure management should be considered in LAA-type MIS patients, but excessive blood pressure reduction leading to hypoperfusion injury should be avoided. Diabetes was also considered a risk factor for LAA. It has been reported that the relationship between diabetes and MIS caused by LAA is stronger than that between diabetes and other MIS subtypes. The prevalence of diabetes in LAA patients is 29% [6]. Chronic hyperglycemia leads to endothelial cell dysfunction, inflammation and accelerated atherosclerosis, resulting in stenosis or occlusion of large vessels [17–21] due to increased oxidant production, lipoprotein enrichment and the formation of advanced glycation end products. High blood sugar can also damage endothelial cells and increase the adhesion of blood-forming components, leading to circulatory dysfunction and exacerbating ischemia. In addition, high blood sugar can also accelerate the aging process of red blood cells, causing them to accumulate in the infarcted area, ultimately promoting the development of stroke. Glucose can enter neurons and alter osmotic pressure, leading to osmotic edema and increasing the incidence of irreversible neuronal damage [22]. This study revealed that a history of diabetes and impaired glucose tolerance were risk factors for vascular stenosis in patients with LAA-type MIS. This showed that a history of diabetes and poor control of fasting blood glucose played a crucial role in the development of vascular stenosis. Therefore, blood glucose should be regularly detected in patients with impaired glucose tolerance or diabetes during follow-up. In addition, this study revealed that compared to MIS patients without a history of ischemic stroke, MIS patients with a history of ischemic stroke had a greater probability of developing CAS. A possible mechanism is as follows: 1) the occurrence of previous stroke was caused by stenosis or occlusion of large blood vessels, and poststroke vascular damage has incomplete reversibility; 2) the occurrence of stroke was caused by the detachment of unstable plaques but timely use of statin drugs to stabilize plaques after stroke. Although the condition has not progressed, high-risk plaques still exist, so the risk of redetachment is greater. Therefore, clinicians need to pay more attention to MIS patients with a history of ischemic stroke. More importantly, this study revealed that lipoprotein-associated phospholipase A2 was an independent risk factor for cerebral artery stenosis in MIS patients. Previous studies have also revealed a strong relationship between cerebral small vessel disease and systemic inflammation, such as high-sensitivity C-reactive protein, and vascular inflammatory markers, such as Lp-PLA2, which may trigger an inflammatory cascade reaction by disrupting the blood-brain barrier [23–26]. Using a rat model of cerebral small vessel disease, some scholars found that white matter lesions were mainly characterized by an increase in reactive astrocytes and activated microglia and significant increases in inflammatory indicators such as tumor necrosis factor-α and Lp-PLA2, which are related to the disruption of the blood-brain barrier were found [27]. After treatment with the inflammatory response inhibitor minocycline, the size of white matter lesions was significantly reduced, cerebral blood flow was improved, and lifespan was prolonged [28]. Therefore, specific vascular inflammatory responses represented by Lp-PLA2 may be an important cause of cerebral artery stenosis in patients with mild ischemic stroke.
The ROC curve of lipoprotein-associated phospholipase A2 in predicting cerebral artery stenosis in patients with atherosclerotic MIS is presented in the figure below (Figure 3).
The ROC curve parameters of lipoprotein-associated phospholipase A2 in predicting cerebral artery stenosis in patients with atherosclerotic MIS were: the area under the ROC curve was 0.700, the standard error was 0.030, the 95% CI confidence interval was 0.636–0.753, and p < 0.0001. The sensitivity was 0.827, and the 1-specificity was 0.470. Therefore, by drawing the ROC curve, the optimal cutoff threshold of lipoprotein-associated phospholipase A2 in predicting cerebral artery stenosis in MIS patients was found to be 69. Therefore, when the level of lipoprotein-associated phospholipase A2 exceeded 69 ng/ml, MIS patients were more likely to develop cerebral artery stenosis. What is more, we found that as lipoprotein-associated phospholipase A2 increased, MMSE decreased and cognitive impairment was more severe in minor ischemic stroke patients. The above research results have not yet been reported by experts in the field of neurology. This new discovery would be beneficial for early and rapid blood tests in patients with mild ischemic stroke to detect and identify the risk of stroke and cognitive impairment. In recent years, some researchers have also found that lipoprotein-associated phospholipase A2 was associated with mild cognitive impairment after stroke and cognitive impairment after microbleeds [29, 30]. This indirectly supports the results of this study to some extent.
This study did not find significant differences in sex, age, hyperlipidemia history, atrial fibrillation history, smoking history, SBP, DBP, TG, TC, LDL-C, VLDL-C, HDL-C, UA, CRP, or HCY levels between the CAS and non-CAS groups. This may be due to the retrospective nature of this study. Although patient-related auxiliary examinations are relatively complete, some patients still have not undergone relevant risk factor assessments (such as alcohol consumption), and there may be deviations in the recording of previous medical history (such as smoking cessation and number of cigarettes smoked). When comparing and analyzing the results, directly treating these patients as missing values or recording inaccurate accuracy may bias the classification results and statistical results. Previous studies have shown that [23] a history of atrial fibrillation is a major risk factor for CAS in patients with cardiac embolism but has a limited role in the formation of CAS in patients with LAA-MIS. In addition, Liu et al. [30] reported no correlation between the levels of TG, TC, LDL-C and vascular stenosis.
In summary, the major etiology of MIS according to the TOAST classification system was LAA. Hypertension history, diabetes history, ischemic stroke history, elevated fasting glucose levels and elevated lipoprotein-associated phospholipase A2 levels were vascular risk factors for LAA-type MIS, while hypertension history and elevated lipoprotein-associated phospholipase A2 levels were independent risk factors for CAS formation. Therefore, clinicians should classify MIS patients using the TOAST system in a timely manner and pay attention to the management of blood pressure and blood sugar, as well as patients with a history of stroke. At the same time, biological indicators such as lipoprotein-related phospholipase A2 should be tested in a timely manner to predict the occurrence of the disease and minimize the formation of CAS, preventing stroke recurrence.
Conclusions
The vascular risk factors for LAA-MIS patients included a history of hypertension, diabetes, ischemic stroke, fasting blood glucose and lipoprotein-associated phospholipase A2, among which a history of hypertension and lipoprotein-associated phospholipase A2 were found to be independent risk factors for CAS. There was also a negative correlation between lipoprotein-associated phospholipase A2 and MMSE.