Introduction
Leiomyomas are benign monoclonal pelvic tumours with an estimated lifetime prevalence in different age groups of 25-50% [1]. Approximately 50% of myomas are asymptomatic, and in the remainder it can present with symptoms of heavy menstrual bleeding, pelvic pressure, pelvic mass, or dysmenorrhoea. Menorrhagia is the predominant indication for hysterectomy in women with myoma [2]. The pathogenesis of these tumours is still elusive, but growing evidence advocates that intrinsic abnormalities of the myometrium, abnormal myometrial receptors for oestrogen, and hormonal changes or altered responses to ischaemic damage during the menstrual period may be responsible for the initiation of (epi)genetic changes found in these tumours [3]. Ultrasound is the preferred imaging modality for its diagnosis. Current literature does not support the hypothesis that leiomyosarcoma arises from uterine myoma, and the reported incidence of occult leiomyosarcoma in uterine fibroid specimens is very low at 0.29%. However, in the presence of high clinical suspicion, magnetic resonance imaging or various clinical scoring systems may be helpful in differentiating leiomyosarcoma from myoma [4]. Available options for the treatment of symptomatic fibroid are medical, surgical, and more recent radiological techniques like magnetic resonance-guided, focused ultrasound surgery or uterine artery embolization. The choice of treatment of myoma is based on patient’s age, presenting symptoms, size, location, and the wish to preserve fertility.
Medical management of fibroid has expanded in the last few years from oral contraceptive pills, progesterone, and danazol, to gonadotropin releasing hormone (GnRH) and selective progesterone receptor modulator (SPRM). Oral progestin is associated with many side effects like breakthrough bleeding and sometimes increased myoma size. Levonorgestrel-releasing intrauterine system is associated with high expulsion rate (up to 20%) in women with fibroid uterus and hence is not recommended for use in distorted uterine cavity [5]. Gonadotropin-releasing hormone agonist down-regulates the hypothalamo-pituitary-ovarian axis leading to reduced oestrogen levels, thereby inducing amenorrhoea and reduction in fibroid size. However, chronic hypoestrogenism with GnRH use is associated with increased incidence of climacteric symptoms and loss of bone mineral density, and hence is approved only for short-term presurgical use [6]. Although pre-operative use of GnRH has been associated with loss of plane of myoma dissection. Myoma tissue exhibits higher concentration of progesterone receptors (PR) A and B compared to normal myometrium [7]. Progesterone promotes proliferation and inhibits apoptosis of myoma tissue through PR [8]. This knowledge has led to the utilization of SPRM in the management of symptomatic myoma. Selective progesterone receptor modulator acts as an antagonist on PRs in myometrium, endometrium, and the hypothalamo-pituitary axis. Its action on the hypothalamo-pituitary axis leads to inhibition of ovulation without altering the serum oestradiol (E2) level [9]. Thus, its use is not associated with hypoestrogenic side effects as seen in GnRH use. Direct effect on endometrium induces benign changes termed as PR modulator-associated changes (PAEC), which usually regress on cessation of treatment [10]. Many previous studies have proven the role of ulipristal acetate (UPA) in decreasing the size of myoma as well as associated symptoms like heavy menstrual bleeding [11–13]. Previous studies have also demonstrated better bleeding control, shorter time to amenorrhoea, and minimal effect on bone mineral density with UPA use compared to GnRH analogue [11, 14]. However, previous trials included mostly Caucasian women who were racially different from the Indian population. In addition, only few studies evaluated the impact of UPA therapy on vascular indices of fibroid. Therefore, we aimed to evaluate the efficacy and safety of 2 repeat cycles of 12 weeks of UPA in symptomatic fibroid along with its impact on vascular indices of fibroid among Indian women.
Material and methods
This longitudinal prospective study was conducted in a single centre at the Department of Obstetrics and Gynaecology, AIIMS, Patna from March 2020 to May 2021. This study was conducted after obtaining approval from the institute’s ethical review committee (AIIMS/Pat/IEC/2019/336). All premenopausal women aged 18-45 years, who had at least 1 symptomatic fibroid of size ranging from 1 to 10 cm as assessed by sonography, with abnormal uterine bleeding, were include in the study. Symptomatic fibroid was defined as the presence of symptoms like heavy menstrual bleeding, pelvic pressure, or pelvic mass.
Exclusion criteria were endometriosis, pelvic inflammatory diseases, endometrial hyperplasia, genital cancer, breast cancer, hemoglobinopathy or severe coagulation disorder, positive pregnancy test at screening, breast-feeding, or liver dysfunction.
All women who fulfilled the inclusion criteria were included in the study. Informed written consent was obtained from each participating individual, and all the procedures were performed in accordance with the institute’s ethical review committee and with the declaration of Helsinki. At the first visit, women were assessed clinically for the reported symptoms and scanned for the following parameters: uterine volume, endometrial thickness and vascularization, and fibroid location, volume, and vascularization. Menorrhagia was assessed using the pictorial blood loss assessment chart score (PBAC), and a score of more than 100, corresponding to > 80 mL of blood during first 8 days of menses, was considered as menorrhagia [15]. Uterine and fibroid volume were calculated using the ultrasound device software formula. In cases with multiple fibroids, the mean volume of up to 3 fibroids was measured. Uterine vascularization was analysed using a power Doppler waveform study and demonstrated as the pulsatility index (PI) and resistive index (RI). To minimize intra-observer variation, the uterine Doppler signal was always obtained from near its origin from the iliac artery and the fibroid Doppler signal was obtained from a core artery (circumferential vascular pseudocapsule). Scanning was performed with a transvaginal probe of the Medison Accuvix A30 Ultrasound System by the same sonographer.
All participants received 5 mg of UPA orally for 12 weeks starting from the 5th day of their menstrual cycle (first treatment cycle). They were instructed to stop consuming UPA for 8 weeks (two menstrual cycle) and then to restart the treatment course for 12 weeks at the end of second menstrual cycle (second treatment cycle). They were asked to note down their daily bleeding pattern in a diary using the PBAC scoring system. They were called up for the second visit at the end of first treatment cycle, the third visit at the end of the second treatment cycle, and the fourth and fifth visit at 3 months and 6 months after completion of the second treatment cycle, respectively.
At each visit the aforementioned clinical and radiological assessments were done. Liver function tests were performed before starting the treatment (baseline) and then at the end of first and second treatment cycles. Endometrial biopsy was performed at baseline and at the end of first and second treatment cycles.
The primary efficacy was measured in terms of time to amenorrhoea and percentage of women who achieved amenorrhoea (PBAC < 2) for the last 35 consecutive days during first treatment cycle. Secondary efficacy was measured in terms of reduction in uterine and fibroid volume as well as its vascularity at the end of the first and second treatment cycles.
Safety assessment included any adverse effect reported during each treatment cycle or off-treatment duration, abnormality of liver function, serum E2 level, or endometrial pathology.
Statistical analysis
Data were analysed using online MedCalc Statistical software version 19.2.6 (MedCalc Sofware bvba, Ostend, Belgium; http://www.medcalc.org; 2020). Continuous variables were expressed as mean ± SD and median with 95% confidence interval. The time to achieve amenorrhea was estimated using the Kaplan-Meier survival analysis with endpoint censored at day 50. Rate of change of fibroid and uterine volume induced by the UPA treatment were assessed by repeated measure analysis of covariance using group and time interaction variables. Safety assessments were based on the safety population, defined as all patients who received 1 or more doses of the study drug, and were analysed using n (%). P < 0.05 was considered statistically significant.
Results
Ninety-four women who fulfilled the inclusion criteria were selected for the ulipristal therapy. However, only 86 women completed the first treatment cycle and attended the second visit. Two patients left the treatment course before completion due to continued spotting and opted for surgical management. Six were lost to follow-up. Only 65 women could complete the second treatment course because the drug was banned in November 2020 by the Drug Controller General of India (DCGI) on the recommendation of the European Medicines Agency (EMA). So complete data for 2 consecutive courses were available for 65 women.
Mean age was 36.1 ± 6.5 years, and mean body mass index was 23.4 ± 3.1 kg/m<sup>2</sup>. Baseline demographic and clinical characteristics of the studied population are summarized in Table 1.
Table 1
Baseline demographic and clinical characteristics of the studied population
| Age (years) mean ± SD | 36.1 ± 6.5 |
|---|---|
| BMI (kg/m2) | 23.4 ± 3.1 |
| Menstrual blood loss | 386 ± 238 |
| Uterine volume (cm3) | 292.1 ± 103.6 |
| Fibroid volume (cm3) | 51.4 ± 54.5 |
| Haemoglobin (gm/dl) | 9.4 ± 1.4 |
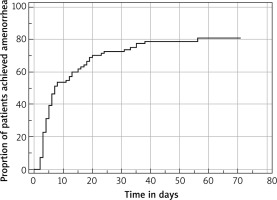
Table 2 summarizes the efficacy results. Seventy-nine per cent of women achieved amenorrhoea for the last 35 consecutive days during the first treatment cycle. The median time to amenorrhoea was 7 days (5 to 15) during the first treatment cycle (Fig. 1). The median time to recovery of menses was 24 days (19–26). These efficacy endpoints were maintained also during the second treatment cycle. A significant response of UPA in terms of fibroid volume reduction was observed, and this continued in the second treatment cycle. The percentage reduction in the mean fibroid volume was 32% after the first treatment cycle and 52% after the second treatment cycle. This reduction was sustained even after cessation of the drug, as noticed at the 4th and 5th follow-up. We observed an increase in fibroid vascular indices (PI and RI), suggesting a reduction in fibroid vascularity. Although an increase in mean uterine artery RI and PI was observed, it did not reach statistical significance.
Table 2
Efficacy endpoint results
The serum E2 level remained at mid-follicular level during the treatment courses. Serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT) did not change significantly (Table 3). Hepatotoxicity based on prespecified criteria (3-fold rise of SGPT/SGOT from the upper limit of normal or greater, 2-fold rise of total bilirubin from the upper limit of normal or greater, and alkaline phosphatase less than 2 times the upper limit of normal) was not observed in any of the patients during either of the treatment cycles. A rise in haemoglobin (Hb) was seen in 51% of women with low baseline Hb.
Table 3
Safety analysis results
During the first treatment cycle drug-related adverse effects occurred in 32% of women; most common were constipation, headache, nausea, hot flushes, and menorrhagia (Table 4). Endometrial hyperplasia was found in 3 women at the end of the first treatment cycle. All biopsies and histopathologies returned as benign. Serious adverse events (anaemia, pain abdomen and dizziness) occurred in one patient during an off-treatment period, but it was not considered as treatment related.
Table 4
Adverse events during and after treatment course 1 and 2
During the second treatment cycle drug-related adverse effects were observed in 38% of women; most common being hot flushes, seen in 7.1%. Four women developed endometrial hyperplasia following the second treatment cycle, but all were histologically benign. No serious adverses event or deaths occurred during treatment or off-treatment in the second cycle.
Discussion
In this trial, 2 intermittent courses of 5 mg UPA were found to be effective in controlling heavy menstrual bleeding and growth of the fibroid. The rate of amenorrhoea for 35 consecutive days was 79% during the first treatment cycle, and those who achieved amenorrhoea did so within 7days of starting the treatment. This efficacy remained maintained during the second treatment cycle; 82% of women achieved 35 consecutive days of amenorrhoea, and median time to amenorrhoea was 5 days. Menstruation was resumed in 96% of women following cessation of treatment, and median time to recovery was 24 days (19–26) and 26 days (20–29) at the end of first and second treatment cycle, respectively. These findings are in concordance with previous studies [12, 16, 17]. Selective progesterone receptor modulator induces amenorrhoea by inhibiting ovulation through the hypothalamo-pituitary axis as well as direct action on endometrium. There was a mild increase in oestrogen level from baseline during the treatment courses because the baseline measurement was taken at the post-menstrual phase whereas other measurements were performed at the end of the treatment cycle before menstruation. The oestrogen level maintained at the early follicular phase level and did not show any flare up during the treatment cycle, which might be the reason for early achievement of amenorrhoea and absence climacteric symptoms. Due to controlled bleeding during the treatment course, a rise in Hb level was seen among the women with low baseline Hb levels.
This study showed a 32% reduction in fibroid volume after the first treatment cycle and 52% after the second 12-week treatment. This reduction was sustained up to the 6-month follow-up. Significant reduction (14%) in uterine volume was also noted after the first treatment cycle, and it continued to decline during the second treatment cycle. The reduction rates were similar to those observed in previous studies [12, 17–19]. Ulipristal acetate has anti-proliferative, anti-fibrotic, and pro-apoptotic effects on the fibroid [20]. Ulipristal acetate is proposed to mediate its action through upregulation of p21 and p27, resulting in cell cycle delay and causing extracellular matrix constriction via stimulation of matrix metallopeptidase 2 expression [21]. This might be responsible for the continued efficacy and reduction in size of fibroid during and after the treatment course. In addition, we found a significant increase in vascularity indices of the core artery following a repeated cycle of UPA treatment, suggesting reduction in blood supply of fibroid, which might be contributory to fibroid shrinkage. Similar findings were reported by Baggio et al. [22]. Although the mean RI and PI of uterine artery were increased after treatment cycle, they did not reach the statistical significance. This suggests that UPA has a greater effect on fibroid vascularity than uterine vascularity.
Although we did not observe any case of severe hepatic injury in our trial, few cases of hepatic injuries have been reported following UPA treatment, and so the EMA committee, responsible for assessing the safety of human medicines, on 12 March 2020, recommended the suspension of the use of UPA for the treatment of fibroids, while a review of its overall safety was in progress. In November 2020, the DCGI also suspended the UPA license for the treatment of fibroids.
Selective progesterone receptor modulator acts directly on the endometrium and has the potential to induce benign histological changes, termed as PAEC. This had been reported to be reversible following cessation of the drug [23]. Few cases of PAEC were detected in this trial, in concordance with previous trials [18, 19]. Few cases of endometrial hyperplasia were noted in our study, which returned as benign on histology. Furthermore, post-treatment endometrial thickness did not differ much from baseline, suggesting no adverse effect on the endometrial lining. No serious drug emerging adverse effect was observed in this trial. The most common adverse effects reported in this trial were nausea and hot flush, which is in accordance with previous studies [18, 19, 23].
There are certain limitations of this study. This study was conducted on small sample size and only short-term follow-up data of up to 6 months following cessation of drugs were available. In addition, this study did not include active comparators like GnRH or mifepristone. We studied effect of only 2 repeat 12-week treatment courses, so data on long-term use of UPA was not known for Indian women. However, a previous study on European women proved the efficacy of extended long-term use (up to 8 cycle) of UPA [24]. A further confirmatory study to evaluate the efficacy and safety of long-term use of UPA in Indian women is required.
Conclusions
In this trial 2 repeat cycles of UPA was found to be safe, well tolerated, and effective in reducing fibroid-related menorrhagia and fibroid size. During treatment cycle, oestrogen levels were maintained at mid-follicular level, thus avoiding the side effect of hypoestrogenism. Although the European Medicine Agency safety committee suspended the use of UPA in view of some reported liver injuries, this trial did not raise any such safety issues. Hence, we recommend intermittent use of UPA in the treatment of symptomatic fibroid.