Summary
In patients undergoing peripheral vascular interventions bivalirudin can be a valid option for anticoagulation during procedure. Impact of bivalirudin on decreased number of bleeding complications and low risk of thromboembolic events, can translate to increased safety of percutaneous peripheral interventions and can reduce cost of hospitalization and ambulatory care of patients with peripheral artery disease.
Introduction
Bivalirudin is an anticoagulant drug, a reversible direct thrombin inhibitor, used mostly in percutaneous coronary interventions (PCI) [1]. The pharmacological profile with a short half-life (around 25 min) makes bivalirudin an option for patients with a contradiction for unfractionated heparin (UFH) or high risk of bleeding [1, 2]. Patients with peripheral artery disease (PAD) can usually be considered as a high-risk group for hemorrhagic events [3]. According to ESC guidelines bivalirudin can be used as the primary anticoagulation in patients with a history of heparin-induced thrombocytopenia [4–6]. Due to the reported lower bleeding complication rate compared to UFH in patients with high risk of hemorrhagic it is worth considering bivalirudin administration [4]. Also, in patients with non-ST-elevation myocardial infarction (NSTEMI) bivalirudin is an alternative to UFH administered combined with GP IIb/IIIa during PCI [5]. Nowadays, indications for bivalirudin are well defined in guidelines for coronary interventions, but there is still a lack of randomized trials focused on anticoagulation during peripheral procedures [6].
Aim
Thus, we aimed to investigate the safety of bivalirudin vs. UFH in percutaneous peripheral interventions (PPI) in short- and long-term follow-up, especially risk of bleeding and thromboembolic complications and major cardiovascular and peripheral events.
Material and methods
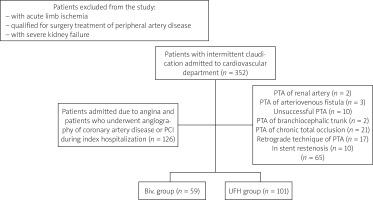
In our retrospective single-center, observational study all data were gathered between 2006 and 2014. One hundred sixty-one patients who underwent PPI were included. The study included patients who underwent different types of PPI: in carotid arteries, in arteries above the knee, in arteries below the knee, in subclavian arteries. We excluded patients after PPI of renal arteries, arteriovenous fistula, in-stent restenosis or with chronic total occlusion. Patients with acute limb ischemia or qualified for surgery of peripheral artery disease were excluded from the study (Figure 1).
Figure 1
Plot: patient inclusion algorithm
Biv. – bivalirudin, PCI – percutaneous coronary intervention, PTA – percutaneous transluminal angioplasty, UFH – unfractionated heparin.
Patients were divided into two groups according to the anticoagulant used during PPI: unfractionated heparin (UFH group) or bivalirudin (Biv. group). Baseline clinical data were assessed. Before the index procedure routine laboratory tests were performed. Procedures were performed in compliance with a standardized institutional protocol. Dosage of UFH was adjusted to patients’ weight. Bivalirudin was administered as a bolus before the PPI and continued as intravenous infusion during the procedure (0.75 mg/kg bolus and 1.75 mg/kg/h infusion) under activated clotting time control. Procedures were performed with the antegrade technique. Vascular sheets were removed 4 h after the procedure and hemostasis was achieved by manual compression. After the procedure and during in-hospital observation patients were assessed for major cardiovascular events (MACCE) such as all-cause mortality, myocardial infarction, stroke/transient ischemic attack, urgent PCI or coronary artery bypass grafting; bleeding complications (classified according to BARC criteria), and for major peripheral events (MAPE) such as vascular complications (bleeding from access site, thrombosis, hematoma, vessel perforation, arteriovenous fistula, pseudoaneurysm) and amputations. In long-term follow-up patients were observed for MACCE, rePPIs, amputations and deaths. The follow-up of the patients was conducted up to 5 years. Data from the Polish National Health Fund were used.
All procedures performed in this study were in accordance with the ethical standards and complied with the Declaration of Helsinki for medical research.
Statistical analysis
Results were presented as number of patients (percentage), mean value with standard deviation (SD) or median with interquartile range (IQR), as appropriate. For dichotomous variables the c2 test and Fisher’s test were used. In the case of dichotomous variables, the Mann-Whitney U-test was used. The Kaplan-Meier method was used to assess the difference in mortality during follow-up between patients. Additionally, multivariable regression analysis (Cox’s regression) was performed to find predictors of long-term mortality. All tests were two-tailed, and a p-value of < 0.05 was considered statistically significant.
Results
We included 161 patients with established indications for PPI. The UFH group consisted of 102 patients (80% men), and the bivalirudin group (Biv. group) consisted of 59 patients (76.2% men). Demographic data are shown in Table I. Mean age of patients was 65.4 ±7.2 years in the Biv. group and 65.1 ±8.5 years in the UFH group. Rates of cardiovascular risk factors were similar between the groups except oral treatment of diabetes (in the UFH group it was higher, p = 0.01). Nineteen (32% in Biv. group) vs. 18 (17.8% in UFH group) percutaneous interventions in carotid arteries were performed; 37 (62.7% Biv. group) vs. 75 (72% in UFH group) in arteries above the knee, 2 (3.4% Biv. group) vs. 7 (6.9% UFH group) in arteries below the knee, and 1 in subclavian artery intervention (both groups). The most common access site was the femoral artery: 31 (52.5%) in the Biv and 79 (49%) in the UFH group.
Table I
Demographic and clinical characteristics
In-hospital observation
Mean in-hospital stay was 7.3 ±4.8 (Biv) vs. 5.9 ±3.8 (UFH) days (p = 0.06). One death in the Biv. group was registered (0.63% Biv, p = 0.18). We observed 12 hematomas at the puncture site (0.63% Biv vs. 7.05% UFH, p = 0.04), 2 pseudoaneurysms (1.27% UFH, p = 0.29), 1 case of thrombosis (0.63% UFH, p = 0.45), and 1 case of bleeding from the puncture site (0.63% UFH, p = 0.45). Arteriovenous fistula, retroperitoneal bleeding and loss of limb were not observed during hospital observation. There were no statistically significant drops of hemoglobin levels in any group (Table II). The total number of hemorrhagic complications was 1.24% in the Biv. group and 8.07% in the UHF group (p = 0.04, Table III).
Table II
Laboratory tests before and after procedure
Table III
In hospital complications, long-term follow-up data and results of regression analysis
Long-term follow-up
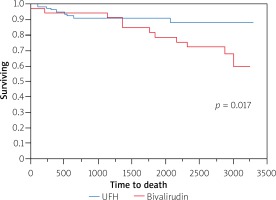
Mean time of long-term follow-up was 65.7 ±36.4 months. All-cause mortality was higher in the Biv. group (8.69%, vs. 7% in UFH group, p = 0.009; Figure 2). The repeated PPI rate was higher in the Biv. group (6.8% vs. 11.8% in Biv. group). In the logistic regression model, with multiple independent variables bivalirudin administration was associated with higher risk of all-cause mortality than UFH (OR = 15. 95% CI: 3.3–107.8; Table III, Figure 2).
Discussion
Bivalirudin is a well-known anticoagulant, used during percutaneous procedures, especially coronary interventions [1]. We confirmed a lower rate of periprocedural complications in bivalirudin with the rate of all in-hospital complications at 1.24%, which makes our results favorable compared to already published data. Usage of bivalirudin was not associated with any ischemic events, such as stroke, acute coronary syndrome (ACS) or thrombosis during in-hospital observation. We reported one case of early thrombosis in the UFH group. However, long-term follow-up showed a higher rate of deaths in the bivalirudin group (8.69% Bi, p = 0.0095). These data were confirmed in our long-term follow-up. It is difficult to discuss that results, due to lack of randomized trials or large reports about safety of bivalirudin in peripheral interventions. Therefore, we can only extrapolate the results from published trials in patients who underwent coronary angioplasty.
In the REPLACE-2 trial (patients with stable CAD) the anticoagulant effect of bivalirudin combined with GP IIb/IIIa was comparable to UFH combined with GP IIa/IIIa [7]. Based on the ISAR-REACT 3 trial, bivalirudin is associated with a lower rate of bleeding events in patients with stable CAD with no influence on mortality during observation [8]. Also, in the ACUITY study bivalirudin (in 7.4% cases with GP IIb/IIIa) versus UFH plus GP II/IIIa inhibitors was favorable in combined ischemic and hemorrhagic complications in 30 days’ observation (p = 0.02). In hemorrhagic complications alone, this difference is more visible in 1-year observation (3.0% vs. 5.7%; p < 0.001) [9]. The ISAR-REACT 4 study (patients with NSTEMI) showed a low rate of serious bleeding events in the group of patients with bivalirudin (2.6% vs. 4.6% of UFH) [10]. The results of the HORIZONS-AMI study showed that treatment with bivalirudin (vs. UFH plus GP IIa/IIIa inhibitors) results in lower rates of cardiac mortality (1.8%, p = 0.03) and all-causes deaths (2.1%, p = 0.047) in 30 days’ FU [11]. It also confirms reductions of major bleeding events in 1-year observation [11]. However, in the HORIZON-AMI study and EUROMAX study the problem of acute in-stent thrombosis was reported [11, 12]. Frequency of stent thrombosis was higher in the bivalirudin group than in the control group (1.6% vs. 0.5%; p = 0.02), with a significant difference within the first 24 h and without a difference in subacute stent thrombosis at 30 days of follow-up [12]. Despite all these results, UFH iv is still a standard anticoagulant in PCI. In the Bravo-3 trial in patients with PAD and severe aortic stenosis, at 30 days of follow-up, use of bivalirudin during transcatheter aortic valve implantation was not associated with reduction of cardiovascular events or bleeding events [13]. Bivalirudin use nevertheless was associated with higher prevalence of acute kidney failure (p = 0.03) [13]. Pseudoaneurysms are common complications after artery puncture during endovascular procedures. The frequency of femoral pseudoaneurysm varies between 0.5% after a diagnostic coronary procedure and 8% after endovascular coronary interventions. Risk of this vascular complication is greater in patients with atherosclerosis, hypertension, obesity, or kidney failure [14–16]. We observed a frequency of 1.27% for pseudoaneurysms after PPI in the group of patients treated with heparin and none in the bivalirudin group. Compared to results of patients after PCI treated with bivalirudin alone by Ormiston el al., hematoma occurred less frequently after PPI than after PCI – 2% vs. 0.63% in our research [17].
Though patients with coronary artery disease have the same risk factors as patients with PAD and percutaneous procedures have some similarities, it is worth pointing out that peripheral procedures are usually longer than PCI and result in a higher rate of bleeding complications, and the risk in patients with PAD is higher than in patients without PAD [3, 18]. That makes studies of PPI relevant and necessary.
The bleeding risk in patients undergoing PPI of lower limb arteries associated with anticoagulant used during the procedure is not well defined. Abtahian et al. presented comparable results of bleeding events in both groups during PCI. There were no differences between bivalirudin and UFH in risk of major bleeding (1.8% UFH vs. 2.4% bivalirudin, p = 0.305), or combined major and minor bleeding (4.3% for both). In-hospital observation also shows similar results for rates of death, MI or re-PCI [19]. In published studies bivalirudin seems to be a safe option for patients who underwent PPI. Rates of major ischemic and adverse bleeding events are low: cerebrovascular events (0.3%), acute renal failure (0.3%), major bleeding (0.8%), distal embolization (3.0%), access site complications (0.5%), minor amputation (0.5%) or bleeding event requiring red blood cell transfusions (0.9%, p = 0.01) [20]. According to Oritz et al., patients who underwent percutaneous vascular interventions with bivalirudin usage have a lower rate of local bleeding complications (access site hematoma) (2.4% vs. 3.9%, p = 0.018) and shorter time of hospitalization (1.0 vs. 1.2 days, p < 0.001) [21]. There are no differences in access site occlusion, distal embolization or mortality. A study by Shammas et al. resulted in an overall complication rate of 4.2% with bivalirudin vs an event rate of 9.2% for UFH during PPI [22]. Sheikh et al. found no differences in procedural success or major and minor bleeding. In contrast to the results of Ortiz et al., Sheikh et al. and our study did not confirm reduction of length in hospital stay [23]. Moreover, the APPROVE trial defined predictors for bleeding events in patients treated with bivalirudin as the primary anticoagulant, such as female gender, exchange to larger sheet and low weight (< 80 kg males, < 62 kg females). The study also showed a low rate of ischemic events, around 1.4%, and a major hemorrhage rate at 2.2% [24]. In our study we did not confirm female gender as a risk factor for bleeding events.
Unfortunately, information about long-term outcomes of bivalirudin in patients undergoing peripheral vascular interventions are limited and trials focus only on 30-day follow-up. The safety profile of bivalirudin in the context of bleeding events is similar in published studies. The mechanisms through which bivalirudin increased mortality in long-term follow-up are unknown and our collected data are insufficient to confirm the exact pathomechanism leading to the suggested higher risk of death. This topic should be further explored in randomized, preferably multicenter trials and confirmed in large-sample size studies.
Conclusions
In patients undergoing peripheral vascular interventions bivalirudin can be a valid option for anticoagulation during the procedure. The impact of bivalirudin on the decreased number of bleeding complications and low risk of thromboembolic events can translate to increased safety of PPIs and can reduce costs of hospitalization and ambulatory care of patients with peripheral artery disease.