Introduction
Sepsis is defined as a dysregulated host response to infection resulting in acute organ dysfunction [1]. Despite the progress made in the clinical management of sepsis, sepsis morbidity and mortality rates remain high. Due to the various functions of the liver in sepsis, liver injury before or after the onset of infection has a crucial effect on the severity and outcome of sepsis in patients [2]. A patient with liver disease (LD) represents an immunocompromised host [3, 4], and therefore, patients with liver failure or chronic liver conditions are not only at higher risk of developing sepsis, but also suffer from increased morbidity and mortality after the sepsis episode [5]. Clinicians should therefore have a high index of suspicion for infection when cirrhotic patients are unwell or present with non-specific symptoms.
The aim of this study was to determine the characteristics, including the use of various diagnostic criteria, outcomes and treatment strategies in septic patients treated outside of the critical care area with pre-existing liver disease.
Material and methods
Study design and participants
Secondary analysis of outcomes for patients with liver disease was performed on a patient population recruited into two annual 24-hour prospective point-prevalence studies on the general wards and emergency departments (ED) across all Welsh acute hospitals in 2016 and 2017. Patient data were collected using an innovative platform developed by the Welsh Digital Data Collection Platform, described in detail in our previous studies [6-8]. In brief, this multi-centre, prospective, observational study of patients with suspected sepsis was conducted in 14 hospitals in Wales with 24/7 consultant-level ED supervision and the facility to admit and treat any acutely unwell patient. We screened patients in the ED and the acute in-patient ward setting with suspected or proven infection on 19th October 2016 and on the 18th October 2017, both Wednesdays (08:00 to 07:59 hours the following day). These dates represented a typically “average” day in the National Health Service (NHS). LD was defined by the treating clinical teams, based on patients’ history, elevated liver enzymes and cross-sectional imaging. In the LD group, all patients had acute or chronic liver problems documented by the medical team. Due to the methodology of the primary study, we did not attempt to further classify this group.
We included patients who fulfilled the following inclusion criteria: patient age of 18 or above, clinical suspicion of infection as documented in the medical notes and National Early Warning Score (NEWS) 3 or above. NEWS is a system for scoring the physiological measurements that are routinely recorded at the patient’s bedside and includes respiration rate, oxygen saturation, systolic blood pressure, pulse rate, level of consciousness or new-onset confusion and temperature [9]. We excluded patients if they were less than 18 years of age or if they were already on intensive care or high dependency units. To facilitate linkage to national databases for the collection of follow-up data, we collected patient identifiable data using the secure data collection tool. We collected data on patient basic demographics, vital sign observations and Systemic Inflammatory Response Syndrome (SIRS) [10], Sequential Organ Failure Assessment (SOFA) [11] and qSOFA (quick SOFA) [12] scores. We also collected microbiological, radiological and laboratory data to facilitate analysis of sepsis source and its complications. Patients were followed up for 90 days for the survival analysis.
We aimed to recruit all eligible patients during the two point-prevalence studies and did not perform any formal sample size calculation.
The project was approved by the South Wales Regional Ethics Committee (16/WA/0071) and patients or legal representatives gave written informed consent. The Defining Sepsis on the Wards project was prospectively registered with an international trial registry (ISRCTN86502304).
Statistical analysis
Categorical variables are described as proportions and are compared using the chi square test. Continuous variables are described as median and range and compared using the Mann-Whitney U test. A two-tailed p-value < 0.05 was considered statistically significant. All statistical tests were calculated using SPSS 25.0 (IBM Corp., Armonk, NY).
Results
Patient characteristics
In our study we screened 12,477 patients over the two 24-hour study periods in 14 Welsh hospitals. 839 patients had NEWS ≥ 3 and documented clinical suspicion of infection and were recruited to the study. Out of all recruited patients, 24 (2.9%) had a past medical history of liver disease. Baseline characteristics are summarised in Table 1.
Table 1
Patient characteristics. Values are number (proportion) or median (range). Comparison between liver disease and non-liver disease cohort was performed using the chi square or Mann-Whitney U test. P values of less than 0.05 are bold
Out of these 24 patients, 4 had biopsy or image proven cirrhosis, 3 had other signs of LD and 1 had signs of mild hepatic encephalopathy. Median Model for End-Stage Liver Disease (MELD) score in the LD cohort was 13.5 (IQR 7-19). The most common comorbidities in the LD group were chronic obstructive pulmonary disease (COPD) and hypertension.
The LD group appeared to have more comorbidities. To clarify this we calculated the comorbidities variables again and compared the number of comorbidities of the LD group with all the comorbidities apart from liver disease. In this setting, the median for both groups was 1 (range: 0-3 for LD patients and 0-5 for non-LD patients, p = 0.6).
Sepsis screening tool analysis
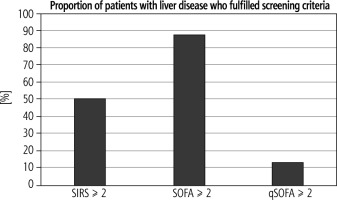
Out of all recruited patients with a history of liver disease, 12/24 (50%) had a SIRS score ≥ 2, 21/24 (87.5%) had a SOFA score ≥ 2 and 3/24 (12.5%) had a qSOFA score ≥ 2 (Fig. 1). Significantly more LD patients than non-LD patients had SOFA ≥ 2 (p = 0.009). Moreover, LD patients had higher total SOFA scores than non-LD patients (Table 2).
Table 2
Comparison of sepsis screening tool scores for liver disease and non-liver disease patients. Comparison between sepsis screening tool scores was performed using Mann-Whitney U test. P value of less than 0.05 is bold
All patients with LD who had an acute (over a period of 24 h) increase in the SOFA ≥ 2 scored in the respiratory, cardiovascular or renal subscores alone, whilst some patients scored on their bilirubin and platelet count values, which could represent the underlying LD.
The difference in median scores for NEWS, SIRS and qSOFA between LD and non-LD patients were not statistically significant (Table 2). Out of all LD patients 15/24 (62.5%) had chest infection, 3/24 (12.5%) urinary tract infection and 11/24 (25%) intra-abdominal infection.
Survival analysis
Out of 839 recruited patients, 222 (26.6%) were non-survivors. The analysis of liver disease cohort revealed that 11/24 (45.8%) of LD patients were nonsurvivors in comparison to 211/815 (25.9%) non-LD patients. Patients with pre-existing liver disease had 2.4 times higher odds (95% CI = 1.07-5.53, p = 0.03) of mortality after the sepsis episode. None of the other comorbidities had a significant impact on the survival (Table 3). There was a higher MELD score in non-survivor LD patients in comparison to LD patients who survived, but it was not significant; median 16 vs. 8, p = 0.07.
Table 3
Impact of comorbidities on patient survival. Data on comorbidities was missing for 36 out of 849 patients. The comparison was performed using chi-square. COPD – chronic obstructive pulmonary disease, IHD – ischaemic heart disease. P value of less than 0.05 is bold
Physiological reserve and sepsis management
We also performed analysis of variables associated with patient characteristics and treatment in hospital to identify differences in patient physiological reserve and sepsis management (Table 4). LD patients were not significantly different than non-LD patients in terms of their frailty (p = 0.78). Both patient cohorts were also not statistically different in regards to do not attempt cardiopulmonary resuscitation (DNA-CPR) order in place (p = 0.06) and ceiling of care (p = 0.54).
Table 4
Comparison of physiological reserve factors in patients with LD in comparison to non-LD patients. Data on selected variables were missing for 38 out of 849 patients Values are number (proportion) or median (range). Comparison between liver disease and non-liver disease cohort was performed using the chi-square or Mann-Whitney U test
| Variable | Liver disease (n = 24) | Non-liver disease (n = 777) | P value |
|---|---|---|---|
| Clinical frailty score, median (range) | 4 (2-9) | 5 (1-9) | 0.78 |
| DNA-CPR, n (%) | 2 (8.7) | 202 (26.0) | 0.06 |
| Ceiling of care, n (%) | 4 (17.4) | 177 (22.8) | 0.54 |
Only 15/24 (62.5%) of septic LD patients received intravenous antibiotics and 8/24 (33.3%) had blood cultures taken. The Sepsis Six pathway was fulfilled for 2/24 (8.3%) and 3/24 (12.5%) patients were seen by a senior clinician. Interestingly, the management was not significantly different from the septic non-LD cohort (p = 0.98, p = 0.79, p = 0.52, p = 0.75 respectively) (Table 5). The most commonly used antibiotic in both cohorts was piperacillin with tazobactam (29.2% of LD patients and 24.8% of non-LD patients).
Table 5
Comparison of management of sepsis patients with LD in comparison to non-LD patients. Data on selected variables was missing for 26 out of 849 patients. Comparison between liver disease and non-liver disease cohort was performed using the chi-square test
Discussion
Infection is responsible for over 50% of admissions of cirrhotic patients to hospitals and is one of the main precipitants for the development of multiple organ failure and death [13]. Our study shows that patients with pre-existing liver disease are at increased risk of mortality after the sepsis episode, despite having similar clinical observations, physiological reserve, received care and being younger than the non-liver disease cohort.
Our data on patient survival are similar to other studies which recruited patients with sepsis outside of the critical care units [14-16]. Liver disease is associated with immune deficiency, which leads to a reduced ability for the organism to prevent or clear infectious agents [17]. It has been suggested that there exists a crucial ‘golden window’ period, during which reversing the acute infective insult and preventing development of organ dysfunction could support hepatic regeneration and facilitate spontaneous recovery [18]. Studies have shown that early diagnosis of sepsis and quick implementation of therapeutic bundles facilitate reduction of the incidence of severe complications and decrease patient morbidity and mortality [19, 20]. The data from our cohort suggest that when multi-organ dysfunction has already developed, such as in most of the patients in the LD cohort, outcomes are particularly poor. It appears that SOFA-based criteria identify most of the patients with liver disease at risk of sepsis, an observation in line with our previous data for all sepsis patients [21, 22]. Whilst SOFA scores can be influenced by chronic stigmata of LD such as low platelet count and high bilirubin, in our cohort patients with LD had an acute (over a period of 24 h) increase of the SOFA score by 2 or more in most cases due to respiratory problems and in some instances due to cardiovascular instability.
Our study also highlights the low rates of antibiotics use, obtaining blood cultures and delivery of the Sepsis Six bundle in the patients with LD who are at increased risk of death from infection [23]. In line with previous reports, the mortality of patients with liver disease is significantly higher than the non-LD cohort despite similar hospital management [24, 25]. This could indicate that patient outcomes are primarily determined by their underlying chronic conditions and that the modifiable effect of treating a potential infection where an organ dysfunction is already established might not significantly change patient outcomes [26]. Appropriate management of the underlying condition, rapid identification of infection and prevention of the development of further organ dysfunction could have the biggest impact on patient survival. However, given the less than adequate delivery of basic sepsis care, it could also be argued that this inappropriate management had a significant effect on the outcomes. As other studies reported similarly poor adherence to the early sepsis bundle elements, it is impossible to establish a causative mechanism from the current data [24].
Further research is required to explore immunological mechanisms that lead to the increased predisposition to infection and worse outcomes in patients with pre-existing liver disease [27]. This could enable development of targeted immunotherapeutic strategies to improve patient survival in well-defined subgroups [28].
The strengths of our study include wide participation of centres including University Hospitals and District General Hospitals and prospectively collected patient information. Our study has high internal validity, as our previous four studies applied the same methodology and recruited a similar number of patients in the same centres [21, 22, 29]. We also obtained a comprehensive dataset and substantial follow-up time [23].
Our study has some significant limitations. The number of patients with liver disease in our cohort is small. However, the proportion of patients with LD is similar to data described in other epidemiological studies [30, 31]. Due to the small sample size, our results should be considered as exploratory, and would need to be confirmed in larger, prospective studies. We were also not able to obtain information about the aetiology of liver disease or establish the cause of death based on the official death certificate. The most common types of liver disease in the UK are alcohol, obesity and viral illness related, and it has been observed that alcohol related admissions are frequent in the Welsh Intensive Care Units [32, 33]. Nevertheless, due to the small size of the cohort, it is unlikely that these data would be beneficial in the analysis of patient mortality [7].
Conclusions
The 90-day mortality was greater in patients with pre-existing liver disease than the rest of the population. It appears that SOFA-based criteria identify most of the patients with liver disease at risk of sepsis, an observation in line with our previous data for all sepsis, despite elements of the SOFA score implicating chronic liver disease. The management of sepsis in patients with liver disease still poses a challenge, with current therapeutic bundles being underused and of unclear significance in improving patient outcomes. It appears that patient survival could be primarily determined by the patient’s underlying condition and could be improved by treatment of the condition and prevention of the infection.