Introduction
Endometriosis is a chronic disease defined by the presence of uterine mucosa (endometrium) outside the uterine cavity. These ectopic lesions contain both endometrial glands and stroma and can be found not only in the peritoneum and in ovaries forming superficial lesions or cysts (endometriomas) but can also infiltrate deep into underlying tissues (deep infiltrating endometriosis – DIE).
Etiopathogenesis of this condition still remains unclear. Although endometriosis affects 10-15% of women in reproductive age, up to 70% of female patients with chronic pelvic pain have been diagnosed with endometriosis and in women with infertility alone, the rate rises to 50%. According to some reports, endometriosis mainly affects women aged 25-29, is more frequent in African Americans in comparison to Caucasians, but, interestingly, is less frequent in women declaring increased alcohol intake. Symptoms of endometriosis are diverse but usually involve: intermenstrual bleeding, painful periods (dysmenorrhea), painful sexual intercourses (dyspareunia), pain during micturition (dysuria) and defecation (dyschezia), and chronic pelvic pain (which often occurs periodically before each upcoming menses). However, the disease can also develop asymptomatically.
Endometriosis is one of the major causes of infertility and thus can result in a serious decline in quality of life [1-3]. An endometriosis classification system has been developed by the American Society of Reproductive Medicine. According to morphology and range of endometrial lesions, staging of endometriosis was divided into 4 types: minimal, mild, moderate and severe [4]. Endometriosis is also a chronic inflammatory and hormone-dependent disease. In particular, oestrogens have been considered to play a crucial role in its development, yet the stage of the disease itself also depends on various genetic, immunological, environmental and endocrine factors. Laparoscopy and subsequent histopathological assessment of specimens still remain the gold standard in the diagnosis of endometriosis. Regrettably, in many symptomatic women the proper diagnosis is often postponed for years, which is mainly attributed to the lack of reliable and dependable non-invasive diagnostic methods.
Several theories have been proposed to clarify the development of endometrial lesions. One of them explains it by the phenomenon of retrograde menstruation (Sampson’s theory). According to it, during menstruation a part of desquamated endometrium gets into the peritoneal cavity via the Fallopian tubes and then undergoes typical hormonal activity transformations according to the cycle phase and, additionally, induces local inflammation [5]. However, retrograde menstruation – which can sometimes be observed during laparoscopy in the perimenstrual period – occurs in the majority of women (up to 90%), but only a small share of them will develop true endometriosis. Cases of endometriosis were also reported in newborns, adolescent girls before menarche and in males, which suggests that other factors other than retrograde menstruation participate in its development [6].
According to another theory, some cells that line the visceral peritoneum are subject to metaplasia under the influence of environmental, inflammatory and hormonal factors. The explanation of this theory reaches back to early embryonal human development and is justified by common origins of peritoneum, pleura, paramesonephric (Müllerian) ducts and ovarian germinal epithelium: the coelom epithelium. Common embryonal origins of peritoneum and endometrium may explain how peritoneal cells can transform freely into endometrial tissue under the influence of some stimulating agents. Moreover, this theory clarifies the ability of endometrial lesions to develop in distant locations, which would be impossible to reach by retrograde menstruation (e.g. lungs) [6]. Recent literature data suggest a role of stem cells in pathogenesis of endometriosis through the ability of their relocation from the basal layer of the uterine mucosa into the peritoneal cavity also by means of retrograde menstruation. Proliferative and cellular differentiation capabilities then allow the stem cells to develop into endometrial implants [6].
Another theory claims that some immunological defects allow the development of endometrial lesions. Several findings seem to support this hypothesis. To begin with, the total macrophage count (which are a source of pro-inflammatory, chemotactic and angiogenic factors) is significantly elevated in peritoneal fluid in patients with endometriosis. Moreover, anti-apoptotic agents themselves can be also identified both in the peritoneal fluid and plasma in these patients. Additionally, the occurrence of immature dendritic cells both in endometrial lesions and in peritoneum in women with endometriosis support the abovementioned hypothesis. It needs to be emphasized that these cells do not appear in the peritoneum in healthy women. The number of mature dendritic cells in both basal and functional layers of the endometrium in women with endometriosis is significantly lower than in those of healthy women. Moreover, some immune disorders may affect NK cell function. Interleukin 6 (IL-6) has already been described as a possible immunosuppressive agent that aims directly at NK cells and their cytostatic properties towards autologous endometrial cells. It has been proven that all the above-mentioned immunological defects facilitate the development of endometrial lesions [6].
The interrelation between endometriosis and chemokines has lately been subject to scientific analysis. Abnormal levels of pro-inflammatory cytokines, growth factors, adhesion molecules, e.g. IL-1, IL-6, IL-8, IL-10, IL-13, IL-15, tumor necrosis factor α (TNF-α), vascular endothelial growth factor (VEGF), monocyte chemoattractant protein-1 (MCP-1), soluble intercellular adhesion molecule-1 (sICAM-1) and metalloproteinases of extracellular matrix (MMP-2, MMP-3, MMP-7, MMP-9), have been found in the endometrium, plasma and also in the peritoneal fluid of patients with endometriosis [6-9]. These soluble agents exert an influence on both development and progression of endometriosis by stimulating cell growth, prompting angiogenesis and triggering endometrial stroma adhesion to the proteins of the extracellular matrix and also by suppressing apoptosis itself. These mechanisms allow for longer survival of endometrial cells in the peritoneal cavity and thus induce growth of endometrial lesions [3, 6-9].
In humans, chemokines are characterized by selective, chemotactic activity towards leukocytes and play a vital role in the process of leukocyte migration. Chemokines are small-cell cytokines (7-15 kDa), which act mostly as extracellular signaling molecules. Those agents mainly originate in lymphocytes, macrophages and monocytes. Both IL-1 and TNF-α stimulate synthesis and secretion of chemokines. Chemokines play a crucial role in the development of primary and secondary immune response, including molecular as well as humoral pathways.
According to the up-to-date literature, more than 50 chemokines and more than 20 chemokine receptors have been identified. Chemokines are divided into four main subfamilies: CXC, CC, CX3C and XC. Three groups (CC, CXC and CX3C) are particularly associated with the development of endometriosis [10-12]. The majority of chemokines are secreted during the inflammatory response, but some of them are produced in a constitutional manner in homeostatic conditions. Moreover, chemokines are involved in the processes of angiogenesis, autoimmunity, neoplasia and metastasis. The chemokine CCL20 (chemokine ligand 20) is named macrophage inflammatory protein (MIP)-3alpha, its activity is regulated by liver cells (liver activation regulated chemokine – LARC), and it belongs to the CC subfamily of chemokines, known as β-chemokines. The CCL20 gene locus is located on the long arm of chromosome 2. In vitro it exhibits strong chemotactic activity towards immature dendritic cells, B lymphocytes and activated T lymphocytes T CD4+ and CD8+. CCL20 is able to trigger rapid adhesion of T lymphocytes to intercellular adhesion molecule (ICAM-1) and endothelial cells stimulated by TNF-α, which suggests its essential role in the extravasation of lymphocytes. CCL20 shows a dual pattern of secretion: its production in the gastrointestinal (GI) tract is not very susceptible to regulation, whereas the liver, skin and lymphatic system secrete it in response to regulatory mechanisms. Depending on the conditions, it may have both homeostatic and pro-inflammatory function. Elevated concentrations of CCL20 were found in HIV-1 carriers, in nasopharyngeal cancer, pancreatic cancer and non-alcoholic fatty liver disease patients [13-16]. The CCR6 receptor (CC chemokine receptor type 6) is the only known receptor for CCL20 and is expressed in the lymphatic system and also outside of it, including the spleen, lymph nodes, appendix, thymus, small intestine, pancreas, and peripheral blood leukocytes – T and B lymphocytes and dendritic cells [17-20].
In a recent systematic review on whether and how chemokines impact the etiopathogenesis of endometriosis 62 papers were investigated [21]. Twenty-seven various chemokines in peritoneal fluid, peripheral blood and eutopic endometrium were analyzed. Statistically significantly (p < 0.05) increased concentrations of chemokines were reported in 55% of analyzed papers. The most frequently investigated chemokines were CXCL8/IL-8 (45.1% papers), CCL2/MCP-1 (37%) and CCL5/RANTES (16.1%). The paper concluded that CXCL8 seems to be the best marker for endometriosis. Its concentration in peritoneal fluid in women with endometriosis was significantly higher than in healthy controls in 15 out of 16 papers (93.75%). However, only 6 out of 13 papers (46,15%) revealed the increased plasma concentration of the abovementioned chemokine. Nevertheless, very few reports on how CCL20 impacts the development of endometrial lesions are available in current literature and the results still seem to be inconsistent. In infertile women with endometriosis no increased CCL20 concentration in peritoneal fluid was identified [22]. On the other hand, the CCL20 chemokine and its receptor CCR6 were found in endometrial ovarian cysts (endometriomas) [23]. Additionally, in the same paper, chemotaxis of Th17 cells induced by CCL20 was found. A review of current literature revealed no papers on the CCL20 concentration in peripheral blood in women with endometriosis.
Aim of the study
The aim of this study was to assess the concentrations of CCL20 in patients with endometriosis and to attempt to determine the impact this chemokine has on the disease.
Material and methods
The study included 32 women of reproductive age who underwent laparoscopy in the Department of Surgical, Endoscopic Gynecology and Oncology, Polish Mother’s Memorial Hospital Research Institute, Lodz, Poland in the timespan from June to September 2018. Patients were divided into 2 groups: study group – endometrial lesions were confirmed during laparoscopy; and control group – no evidence on endometrial lesion during laparoscopy. The study group was then divided into three subgroups according to endometriosis form confirmed during laparoscopy: peritoneal endometriosis, endometrial ovarian cysts (endometriomas) and deep infiltrating endometriosis (DIE). Twenty patients aged 23-47 (mean 33.45) were included in the study group and 12 patients aged 22 to 45 (mean 36.92) acted as controls. Within the study group peritoneal endometriosis was confirmed in 12 cases (60%), endometrial ovarian cysts (endometriomas) in 4 (20%) and DIE in 4 cases (20%). The indications for laparoscopy were: confirmed endometriosis or suspicion of the disease – chronic pelvic pain or infertility. Every patient has provided written informed consent for participation in the study and agreed to have their blood sample collected before surgery. The protocol of the study has been approved by the Ethics Committee of Polish Mother’s Memorial Hospital-Research Institute (Opinion No. 73/2017).
Blood samples were drawn from each patient before the surgery into a serum separator tube. After 30 min of incubation at room temperature the samples were centrifuged for 15 min at 2000 ×g. A part of sera was collected into a 1.5 ml Eppendorf tube and frozen at –80°C. The human CCL20/MIP-3α concentration values were determined using a quantitative sandwich ELISA kit for the quantitative determination of human CCL20 concentrations in cell culture supernates, serum and plasma (R&D Systems, Inc. Minneapolis, MN 55413, USA). The values of CCL20 protein concentrations were determined according to the manufacturer’s protocol. The standard curve concentrations used for the ELISA were 500 pg/ml, 250 pg/ml, 125 pg/ml, 62.5 pg/ml, 31.2 pg/ml, 15.6 pg/ml and 7.8 pg/ml. The detection range of this test was 7.8-500 pg/ml. The minimum detectable dose was 0.47 pg/ml. The standard curve was created using computer software (Gen5 Microplate Reader and Imager Software version 3.02, BioTek) able to generate a four-parameter logistic (4-PL) curve fit. The optical density of each well was evaluated using a microplate reader (Synergy H1 Microplate Reader, BioTek) set to 450 nm (with wavelength correction set to 540 nm). Results were statistically analyzed by SPSS STATISTICS 24.0.0 software using t-test and ANOVA methods. A significance level of 0.05 was used.
Results
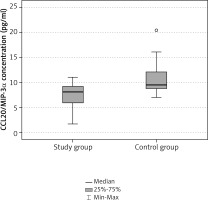
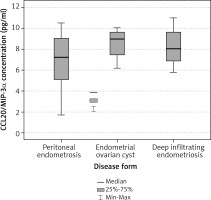
The mean serum concentration of CCL20/MIP-3α in the study group was 7.4 pg/ml and the median was 8.12 pg/ml. In controls the mean value was 10.9 pg/ml, median 9.47 pg/ml respectively (Table 1). The concentration of CCL20/MIP-3α was statistically significantly lower in the study group than in controls (p = 0.004, Table 2, Fig. 1). Within the study group the highest values were observed in patients with endometrial ovarian cysts (8.55 pg/ml), intermediate in deep infiltrating endometriosis subgroup (8.24 pg/ml) and the lowest in patients with peritoneal form of the disease (6.74 pg/ml, Table 3). Differences within the study subgroups were not statistically significant (p = 0.385, Table 4, Fig. 2).
Table 1
Serum level of CCL20/MIP-3α
| Serum level of CCL20/ MIP-3α [pg/ml] | Study group (n = 20) | Control group (n = 12) |
|---|---|---|
| Average | 7.40 | 10.95 |
| Median | 8.12 | 9.47 |
| Q25 | 5.86 | 8.64 |
| Q75 | 9.21 | 12.40 |
| SD | 2.58 | 3.89 |
| Min | 1.71 | 6.95 |
| Max | 11.00 | 20.47 |
Table 2
Comparison of CCL20/MIP-3α serum levels between groups
| Study group (n = 20) | Control group (n = 12) | t-test | ||||
|---|---|---|---|---|---|---|
| Average | SD | Average | SD | t | p | |
| CCL20/MIP-3α serum level [pg/ml] | 7.40 | 2.58 | 10.95 | 3.89 | –3.109 | 0.004 |
Table 3
Serum levels of CCL20/MIP-3α in endometriosis subgroups [pg/ml]
Discussion
Our study is the first to reveal statistically significantly decreased serum levels of CCL20 in patients with endometriosis. Yet, it did not reveal any significant differences in the serum levels of this chemokine with regard to various forms of the disease (peritoneal endometriosis, ovarian cysts and DIE). Although the size of both study and control groups was rather limited (which is an obvious limitation of our study), our findings have clear statistical significance, which enables us to draw strong conclusions. Current literature lacks proper reports on serum CCL20 concentration and its impact on the etiopathogenesis of endometriosis, and at present CXCL8/IL-8 seems to be the best serum marker for endometriosis. Current research efforts designed to extend our understanding of endometriosis development focus on three major chemokines: CXCL8/IL-8, CCL2/MCP-1 and CCL5/RANTES [21]. According to one paper, CCL20 levels in peritoneal fluid of infertile women with endometriosis were not altered [22]. Some reports show the CCL20 expression in both epithelial and stromal cells in endometrial lesions [23]. CCL20 has mainly chemotactic properties aimed at immature dendritic cells (DCs), B lymphocytes and activated T lymphocytes CD4+ and CD8+. Furthermore, CCL20 impacts on extravasation of lymphocytes and is secreted in humans both in constitutional and induced conditions and has both homeostatic and pro-inflammatory properties. Expression of cells which exhibit chemotaxis towards CCL20 was reported in endometrial lesions. There are some available reports on increased density of immature DCs (CD1a+) in the basal layer of endometrium in women with endometriosis. Furthermore, increased density of these cells was noted in peritoneal endometrial implants and peritoneum that was closely adjacent to them [24]. In another study higher expression of T lymphocytes CD4+, CD8+ and polyclonal activation of B cells were detected in peritoneal fluid of women with endometriosis [25]. Also Th17 cell chemotaxis induced by CCL20 in women with endometrial ovarian cysts was reported [23].
The above mentioned analyses proving CCL20 presence in endometrial lesions and lack of its increased concentrations in peritoneal fluid and peripheral blood argue in favor of mainly local aggregation of CCL20 in endometrial implants. Reports on the role of immune cells with substantial chemotactic properties towards CCL20 in the progression of the disease could only further clarify this hypothesis.
In our study, conclusions need to be drawn carefully. Further studies are warranted in order to precisely investigate the role of CCL20 in the etiopathogenesis of endometriosis and clarify this matter, especially in larger groups.
Conclusions
Our study revealed statistically significantly decreased CCL20 serum levels in women with endometriosis. However, in this study no significant differences of serum CCL20 levels between patients with peritoneal endometriosis, endometrial ovarian cysts and deep-infiltrating endometriosis were observed.