Summary
The highlight of our study was that we evaluated the predictive value of serum oxidative stress factors combined with ischemia-modified albumin (IMA) and heat shock protein 70 (HSP70) for the occurrence of myocardial ischemia-reperfusion injury events in patients with acute myocardial infarction (AMI) combined with type 2 diabetes mellitus (T2DM). The findings revealed that superoxide dismutase, malondialdehyde, myeloperoxidase, IMA, or HSP70 level alone had good diagnostic power in patients with AMI combined with T2DM, which was in line with previous studies.
Introduction
Acute myocardial infarction (AMI) results in ventricular remodeling in response to oxygen demand, which is characterized by left ventricular dilatation and elevated myocardial wall stress [1, 2]. Percutaneous coronary intervention (PCI) has been regarded as a gold standard therapy for AMI therapy to restore coronary blood flow [3, 4]. AMI is a frequent reason for fatality in patients with type 2 diabetes mellitus (T2DM) and vascular dysfunction is a main ingredient of diabetic cardiomyopathy [5]. Despite significant improvements in outcomes for the general AMI population, the in-hospital mortality rate in patients with T2DM patients has consistently increased regardless of diabetes status [6]. Therefore, these patients particularly require better treatment options, and it is necessary to pursue intensive medical treatment, prolong the surveillance, and stringently control the risk factors of the disease.
Free radicals interact with biomolecules because of their reactive structure, and oxidative stress has been revealed to correlate with cancer, cardiovascular disease, as well as the progression of the aging process [7]. The degree of oxidative stress is mainly appraised by the levels of malondialdehyde (MDA), superoxide dismutase (SOD), as well as reactive oxygen species (ROS) [8]. MDA, SOD, and myeloperoxidase (MPO) may be considered to be essential parameters for a sign of cell oxidative damage, and their levels reflect the degree of oxidative damage [9]. The properties of albumin can change under ischemic attacks linked to oxidative stress, acidosis, and the production of ROS. Under these conditions, the resulting ischemia-modified albumin (IMA) has a decreased metal-binding capacity, particularly for transition metals [10]. IMA is known as a structurally modified form of albumin, which could serve as a potent and novel screening hallmark for assessing diabetic retinopathy (DR)-associated oxidative stress [11]. Meanwhile, IMA has also been revealed to be a favorable hallmark for acute coronary syndrome (ACS) [12]. Heat shock protein (HSP)70 exerts functions in the pathophysiology of insulin resistance, β-cell dysfunction, and multiple diabetic complications, including impaired hemostasis and micro- and macro-vascular alterations [13]. Elevated circulating HSP70 has been suggested to be a myocardial damage marker after AMI and may also enhance heart failure progression [14]. Evidence has also shown that patients with diabetes have higher HSP70 levels, which may be a parameter of metabolic disorders occurring in the course of diabetes [15]. Based on the above-cited references, it could be concluded that serum oxidative stress factors, IMA, or HSP70 might be vital hallmarks for cardiac-related diseases and diabetes-related diseases.
Aim
The objective of our study was to evaluate the clinical diagnostic value of serum oxidative stress factors (SOD, MDA, and MPO) combined with IMA and HSP70 for myocardial ischemia-reperfusion injury (MIRI) after PCI in elderly patients with AMI and T2DM.
Material and methods
Ethical approval
This study research was ratified by the ethics committee of our hospital (approval number: 20200422), and the patients and their families gave written informed consent for the research.
Study subjects
Ninety-four elderly patients with AMI combined with T2DM requiring PCI who were admitted to our hospital between November 2020 and August 2021 were included in the study (AMI + T2DM group), and 86 patients with AMI without T2DM who were treated with PCI were included as controls (AMI group). The occurrence of MIRI events in the AMI and AMI + T2DM groups was recorded according to whether patients in both groups developed MIRI within 48 h after PCI.
Inclusion criteria: meeting the diagnostic criteria of T2DM in the Guidelines for the Prevention and Treatment of Type 2 Diabetes Mellitus [16]; insulin control and glucose-lowering drug treatment; confirmed by coronary angiography and meeting the diagnostic criteria of AMI in the 2018 ESC/ACCF/AHA/WHF fourth universal definition of myocardial infarction [17]; complete clinical data; no psychiatric disorders, cognitive impairment, or immune system diseases.
Exclusion criteria: previous history of myocardial infarction, history of PCI, intracoronary thrombolysis, and coronary artery bypass surgery; or combined malignancy, serious infectious disease, inflammatory disease, hematologic disease, as well as acute cerebral infarction.
Diagnostic criteria for clinical MIRI: severe reperfusion arrhythmias (including frequent premature ventricular contraction, ventricular tachycardia, ventricular fibrillation, and sinus bradycardia), no recurrent flow and slow flow (Thrombolysis In Myocardial Infarction (TIMI) flow grade 2 or less on post-PCI coronary angiography), severe chest pain, paradoxical ST-segment elevation, and transient hypotension after PCI revascularization [18].
TIMI flow classification referred to the reperfusion of the infarct-related coronary arteries (culprit vessels) in AMI and was usually evaluated by coronary angiography, which was classified into 4 grades: TIMI grade 0: no antegrade flow distal to the vessel occlusion; TIMI grade I: the partial passage of contrast through the occlusion site, but failure to fill the distal vessels; TIMI grade II: complete filling of the distal coronary artery with contrast, but delayed filling and clearance of contrast compared to normal coronary arteries; TIMI grade III: complete and rapid filling of the distal vessels with contrast agent and rapid clearance. TIMI grade 0 and I indicated non-reperfusion in a coronary artery, and TIMI grade II and III indicated coronary artery recanalization (reperfusion).
Treatment methods
Prior to PCI, patients received oral administration of aspirin enteric-coated tablets 300 mg (Bayer Healthcare Company Ltd., China, 0.1 g/tablet), ticagrelor tablets 180 mg (AstraZeneca AB, Sweden, 90 mg/tablet), and atorvastatin 80 mg (Pfizer Ltd., China, 20 mg/tablet).
Emergency coronary angiography combined with percutaneous transluminal angioplasty was performed within 90 min of admission by physicians with clinical and surgical experience in cardiology; the culprit vessel was stented with ≥ 1 coronary stent according to the degree and extent of stenosis, and the angiography showed residual stenosis less than 20% and TIMI grade III for distal flow.
After PCI, patients received oral administration of aspirin enteric-coated tablets 100 mg (once a day), ticagrelor tablets 90 mg (twice a day), and atorvastatin calcium tablets 40 mg (once a day). If the patients had angina symptoms after PCI, they received oral administration of isosorbide mononitrate tablets 10 mg (Livzon (GROUP) Pharmaceutical FACTORY, China, 10 mg/tablet, twice a day) as well as conventional drug therapy for pre-existing underlying diseases [19].
Gensini scoring
During PCI, the specific location and degree of stenosis of the coronary lesion were recorded in all patients. The score was then calculated following the Gensini scale, and it was generally considered that the higher the score was, the more severe was the coronary lesion [20]. The specific scoring criteria are listed in Table I.
Table I
Gensini scoring standards
Sample collection
The venous blood (3–5 ml) was collected from the patient at the time of admission, placed at room temperature for 1–2 h, and centrifuged at 5000 rpm/min for 10 min for collection of the supernatant, which was stored at –80°C for backup.
Parameter measurement
The serum levels of oxidative stress factors (SOD, MDA, and MPO), IMA, and HSP70 were measured in patients by enzyme-linked immunosorbent assay (ELISA), and the kits were obtained from R&D Systems (Minneapolis, MN, USA).
The routine blood count, renal function, cardiac troponin I (cTnI), high-sensitive C-reactive protein (hs-CRP), and other related parameters of patients were evaluated by an automatic biochemical analyzer. All patients had bedside echocardiography completed by two experienced color ultrasound physicians on admission, and the patient’s left ventricular ejection fraction values (LVEF) were recorded.
Statistical analysis
All data were statistically analyzed using IBM SPSS Statistics 21.0 (IBM Corp.) statistical software. Measurement data that conformed to a normal distribution and showed homogeneity of variance were depicted as mean ± standard deviation, and were analyzed by the t-test. Numeration data were expressed as percentages and group comparisons were implemented with Fisher’s exact test or χ2 tests. Univariable or multivariable logistic regression was employed to analyze factors influencing the occurrence of MIRI after PCI in patients with AMI and patients with T2DM combined with AMI. ROC curves were employed to analyze the predictive power of serum oxidative stress factors, IMA, and HSP70 alone or in combination for the development of MIRI after PCI in elderly patients with AMI combined with T2DM or AMI without T2DM. A p-value below 0.05 was considered to be statistically significant.
Results
Baseline data comparison
No significant differences were found in gender, body mass index (BMI), age, smoking history, hypertension history, number of ST-elevation myocardial infarction (STEMI) cases, culprit vessels, and onset to balloon dilatation/stenting time between the AMI + T2DM and the AMI groups (all p > 0.05). The patients in the AMI + T2DM group had higher admission blood glucose levels, white blood cell count, Gensini score, and levels of cTnI, hs-CRP, MDA, MPO, IMA, and HSP70, and lower LVEF and SOD levels in comparison to the AMI group (admission blood glucose levels: p < 0.001; white blood cell count: p = 0.002; Gensini score: p = 0.001; cTnI: p < 0.001; hs-CRP: p < 0.001; SOD: p = 0.034; MDA: p = 0.027; MPO: p = 0.044; IMA: p = 0.006; HSP70: p = 0.006). When comparing the degree of coronary lesions between the two groups, the difference between the groups with three or more and single coronary lesions was statistically significant (single: p = 0.004; three or more: p = 0.001), while the difference between the groups with 2 coronary lesions was not statistically significant (p > 0.05) (Table II).
Table II
Comparison of the general condition of the patients
[i] AMI – acute myocardial infarction, T2DM – type 2 diabetes mellitus, BMI – body mass index, STEMI – ST-elevation myocardial infarction, LVEF – left ventricular ejection fraction, cTnI – cardiac troponin I, hs-CRP – high-sensitivity C-reactive protein, SOD – superoxide dismutase, MDA – malondialdehyde, MPO – myeloperoxidase, IMA – ischemia-modified albumin, HSP70 – heat shock protein 70.
MIRI event comparison
After PCI in all patients, the occurrence of MIRI events in the AMI and AMI + T2DM groups was recorded. There was a higher probability of MIRI events in the AMI + T2DM group (13 cases, 15.12%) in contrast to that in the AMI group (25 cases, 26.60%) (p = 0.043; Table III).
Relevant indicators in the MIRI and non-MIRI groups
In both the AMI and AMI + T2DM groups, patients in the MIRI group had increased MDA, IMA, MPO, and HSP70 levels, number of patients with multibranch coronary lesions and onset to balloon dilatation/stenting time, and reduced SOD levels relative to the non-MIRI group (all p < 0.05). Among them, patients in the AMI + T2DM group who developed MIRI had higher serum MDA, IMA, MPO, and HSP70 levels and lower SOD levels than those in the AMI group who developed MIRI (all p < 0.05). In the AMI + T2DM group, patients in the MIRI group possessed higher admission glucose levels versus those in the non-MIRI group; in the AMI group and the AMI + T2DM group, comparisons of the rest of the clinical and biochemical indicators between the non-MIRI and MIRI patients were not statistically significant (all p > 0.05) (Tables IV, V).
Table IV
Comparison of relevant indicators between the MIRI group and the non-MIRI group of AMI group patients
[i] AMI – acute myocardial infarction, BMI – body mass index, STEMI – ST-elevation myocardial infarction, LVEF – left ventricular ejection fraction, cTnI – cardiac troponin I, hs-CRP – high-sensitivity C-reactive protein, SOD – superoxide dismutase, MDA – malondialdehyde, MPO – myeloperoxidase, IMA – ischemia-modified albumin, HSP70 – heat shock protein 70.
Table V
Comparison of relevant indicators between the MIRI group and the non-MIRI group of AMI + T2DM group patients
[i] AMI – acute myocardial infarction, T2DM – type 2 diabetes mellitus, BMI – body mass index, STEMI – ST-elevation myocardial infarction, LVEF – left ventricular ejection fraction, cTnI – cardiac troponin I, hs-CRP – high-sensitivity C-reactive protein, SOD – superoxide dismutase, MDA – malondialdehyde, MPO – myeloperoxidase, IMA – ischemia-modified albumin, HSP70 – heat shock protein 70.
Multivariable logistic regression analysis of MIRI after PCI in patients with AMI
Statistically significant indicators in Tables IV and V were utilized as independent variables, and whether MIRI occurred after PCI or not was the dependent variable (yes as “1” and no as “0”). Multivariable logistic regression analysis revealed that high levels of MDA, IMA, MPO, HSP70, low levels of SOD, multiple branches of coronary lesions, STEMI, and prolonged time from onset to balloon dilatation/stenting were the risk factors for MIRI after PCI in patients with AMI; high admission glucose levels, high levels of MDA, IMA, MPO, HSP70, low levels of SOD, multi-branch coronary lesions, history of hypertension, STEMI and prolonged time from onset to balloon dilatation/stenting were risk factors for post-PCI MIRI in patients with T2DM + AMI (Tables VI, VII).
Table VI
Multivariate logistic regression analysis of post-PCI MIRI in patients with AMI
Table VII
Multivariate logistic regression analysis of post-PCI MIRI in patients with AMI + T2DM
ROC curve parameters of serum oxidative stress factors plus IMA and HSP70 regarding MIRI events in the AMI and AMI + T2DM groups
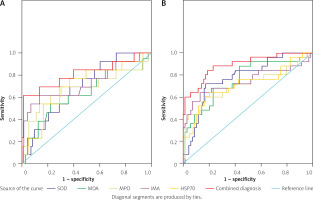
ROC curves were plotted to evaluate the predictive values of serum oxidative stress factors combined with IMA and HSP70 for the occurrence of MIRI events in patients in the AMI and AMI + T2DM groups, respectively. The results showed that SOD, MDA, MPO, IMA, and HSP70 levels in the AMI group had an area under the ROC curve (AUC) of 0.690, 0.665, 0.675, 0.724, and 0.703 (SOD (95% CI: 0.552–0.828, p = 0.030); MDA; MPO (95% CI: 0.485–0.868, p = 0.045); IMA (95% CI: 0.547–0.902, p = 0.010); HSP70 (95% CI: 0.543–0.863, p = 0.020)). SOD, MDA, MPO, IMA, and HSP70 levels in the AMI + T2DM group had an AUC of 0.776, 0.758, 0.710, 0.741, 0.714, and 0.890 (SOD (95% CI: 0.671–0.880, p < 0.001); MDA (95% CI: 0.643–0.874, p < 0.001); MPO (95% CI: 0.571–0.848, p = 0.002); IMA (95% CI: 0.603–0.879, p < 0.001); HSP70 (95% CI: 0.582–0.846, p = 0.002)). As for the combination prediction, the AUC was 0.811 (95% CI: 0.654–0.968, p < 0.001) in the AMI group and 0.890 (95% CI: 0.812–0.968, p < 0.001) in the AMI + T2DM group (Table VIII and Figure 1). It was found that the ROC curve for the combined prediction of SOD, MDA, MPO, IMA, and HSP70 for the occurrence of MIRI events was higher in both the AMI and the AMI + T2DM groups than for predictive factors alone.
Table VIII
ROC curve parameters of serum oxidative stress factors plus IMA and HSP70 regarding MIRI events in the AMI and AMI + T2DM groups
Figure 1
ROC curve parameters of serum oxidative stress factors plus IMA and HSP70 regarding MIRI events in the AMI and AMI + T2DM groups. A – ROC curve of serum oxidative stress factors combined with IMA and HSP70 for MIRI events in the AMI group; B – ROC curve of serum oxidative stress factor combined with IMA and HSP70 for MIRI events in the AMI + T2DM group
Discussion
The prevalence of T2DM in AMI patients is estimated to be approximately 25%, and patients with diabetes have worse short- and long-term prognosis compared with non-diabetics [20, 21]. MIRI appears to be stimulated partly by neutrophil activation, and its underlying mechanisms consist of cell damage resulting from the release of proteolytic enzymes, oxygen free radicals, along with cytotoxic substances; the generation of inflammatory mediators contributes to vascular endothelial cell damage, enhanced vascular permeability, together with edema; subsequent activation of inflammatory cells enhances the inflammatory response [22]. In our paper, we aimed to assess the clinical diagnostic value of serum oxidative stress factors combined with IMA and HSP70 for MIRI in elderly patients with AMI and T2DM. Our primary endpoint was MIRI after PCI in elderly patients with AMI and T2DM, and our secondary endpoint was MIRI after PCI in patients with ACI.
The main findings of our study were that: (1) the elderly patients with AMI combined with T2DM had higher MDA and MPO levels, and lower SOD levels. The generation of oxygen free radicals can destroy the cardiac cell membrane, damage the structure of myocardial cells, and cause secretion of many lipid peroxides [23]. Additionally, oxidative stress can occur in diabetes, and the outcomes of oxidative stress can advance the onset and development of diabetic complications [24]. MDA can reflect the content of the oxygen-free radical and lipid peroxidation index, while SOD can reflect the body’s capacity to resist lipid peroxidation [25]. As previously reported, MDA is elevated in myocardial tissues after MIRI, and reduction of MDA content has a cardioprotective effect [26]. The activation degree of MPO correlates closely with the degree of neutrophil accumulation and infiltration into the myocardial ischemic tissue, whose activity could reflect the extent of neutrophil accumulation and infiltration [9]. Furthermore, evidence has shown that MDA content is increased and the SOD activity is diminished in patients with MIRI [27]. All these findings indicate the value of serum oxidative stress factors in related diseases.
(2) In our study, elderly patients with AMI combined with T2DM had higher IMA and HSP70 levels. Recently, some articles have focused on the importance of HSP70 in cardiac and diabetic-associated diseases. For example, elevated levels of HSP70 have been found in ischemic stroke and AMI patients, and HSP70 might serve as a hallmark for developing novel regimens in the diagnosis and therapy of cerebrovascular and cardiovascular diseases [28]. Song et al. supported the view that HSP70 exhibits a high level during MIRI and plays a cardioprotective role against MIRI through restraining ROS generation, impeding cell apoptosis, as well as mitigating calcium overload [29]. Also, it was reported that elevated circulating HSP70 levels may play a part in gestational diabetes mellitus pathogenesis and may also be a marker of this disease [30]. Similarly, some articles also have focused on the importance of IMA in cardiac and diabetic-associated diseases. For instance, IMA has been demonstrated to be bound up with LVEF and acts as an early parameter of left ventricular dysfunction in patients with STEMI [31]. Chen et al. stated that enhanced oxidative stress can lead to elevated serum IMA levels, and advanced oxidative stress enhances the possibility of coronary collateral circulation formation [32]. Another study revealed higher levels of IMA in T2DM, and inflammation and hyperglycemia decreased the capability of albumin to bind to cobalt, thereby contributing to higher IMA levels [33].
(3) Multivariable logistic regression analysis revealed that high levels of MDA, IMA, MPO, HSP70, low levels of SOD, multiple branches of coronary lesions, STEMI, and prolonged time from onset to balloon dilatation/stenting were risk factors for MIRI after PCI in patients with AMI; high admission glucose levels, high levels of MDA, IMA, MPO, HSP70, low levels of SOD, multi-branch coronary lesions, history of hypertension, STEMI and prolonged time from onset to balloon dilatation/stenting were risk factors for post-PCI MIRI in patients with T2DM + AMI. Similar to our finding, severe admission hyperglycemia can be considered as a prospective high-risk marker for patients with non-diabetic AMI [34]. Hypertension history and diabetes among young patients with AMI increase over time [35].
Conclusions
It was found that SOD, MDA, MPO, IMA, or HSP70 level alone had good diagnostic power in patients with AMI combined with T2DM, which was in line with the above-cited articles. However, our research also demonstrated that the combination of serum oxidative stress factors combined with IMA and HSP70 had greater predictive value for MIRI events after PCI in patients with AMI combined with T2DM. Our study suggests that the combination of serum oxidative stress factors combined with IMA and HSP 70 could serve as an alternative novel biomarker for the estimation of AMI and T2DM. These indicators can be used for clinical auxiliary testing and, if there is a change, can indicate further examination for diagnosis. The highlight of our paper was that we evaluated the predictive value of serum oxidative stress factors combined with IMA and HSP70 for the occurrence of MIRI events in patients with AMI combined with T2DM. Further studies are still warranted to support the findings.








