Introduction
Non-Hodgkin lymphoma (NHL) is the eighth most commonly diagnosed cancer in men and the eleventh most commonly diagnosed cancer in women [1]. In Egypt, lymphoid malignancies account for 10–12% of all malignancies; NHL is the third most common cancer in Egyptian males and the second most common cancer in females [2].
Toll-like receptors (TLRs) are essential receptors of the innate immune system and key regulators of the acquired immune system. They are activated by pathogen-associated molecular patterns or damage-associated molecular patterns [3]. Signal transduction through TLRs induces the expression of various genes required for immune stimulation via activation of nuclear factor κ-B (NF-κB) and mitogen-activated protein kinases (MAPKs) [4].
TLRs play an essential role in B-cell activation and maturation and may be involved in the pathogenesis of B-cell lymphomas through the activation of NF-κB and associated genes, resulting in the production of cytokines and tumour necrosis factor α (TNF-α) [5].
Negative regulation of TLR signalling is important to counteract the over-activation of TLRs and possible subsequent autoimmune damage, where soluble TLRs function as the first-line negative regulatory mechanism [6].
Therefore, the present study aimed to assess the serum levels of sTLR2 and sTLR4 in a group of patients with NHL along with a group of healthy control volunteers and to investigate their correlations with the laboratory and clinicopathological parameters of these patients.
Material and methods
The study included 100 patients with NHL and 50 sex- and age-matched healthy volunteers. Patients were grouped into groups I or II based on the Ann Arbor staging. Group I included 50 patients with stage I or II, while group II included 50 patients with stage III or IV disease. The study was conducted in the Damanhour Oncology Centre from April 2018 to August 2019. Informed consent was obtained from all participants. Ethical approval was obtained from the Ethical Committee of the Faculty of Pharmacy (ref no. 3/8 PB3).
Inclusion and exclusion criteria
All patients involved in the study were diagnosed with NHL. Subjects with other types of malignancies, inflammatory diseases, life-threatening illness, or severe organ dysfunction were excluded from the study. Healthy subjects less than 18 years of age and pregnant and/or lactating females were not included in the study.
Clinical examination
Both NHL cases and the control group were subjected to medical history taking, clinical examination, and radiological assessment. For histopathological diagnosis, a biopsy was taken from patients from sites of malignancy, including lymph nodes, extranodal masses, or spleen and bone marrow in selected cases to confirm the diagnosis and for proper subtyping and staging of disease.
Sample collection
Approximately 5 ml of whole blood was withdrawn from each participant using sterile disposable plastic syringes under complete aseptic conditions and divided into two aliquots: 1 ml was collected in tubes containing K2EDTA for a complete blood count (CBC) and 4 ml was collected in plain tubes and left to clot at 25°C for 30 min, followed by centrifugation at 4000 rpm for 10 min to separate serum. The separated serum was stored at –20°C for determination of serum sTLR2 and sTLR4 levels.
Biochemical analyses
CBC was assessed using the ADVIA 2120 Haematology System (Bayer HealthCare, Diagnostics Division, Germany) [7]. Serum sTLR2 was assessed using a human soluble toll-like receptor 2 ELISA kit (Cat. No. In-Hu4102) (Bioneovan Co., Ltd., Beijing, China) [8]. Serum sTLR4 in serum samples was evaluated using a human soluble toll-like receptor 4 ELISA kit (Cat. No. In-Hu4103) (Bioneovan Co., Ltd., Beijing, China) [9].
Statistical analysis of data
Data were analysed using IBM SPSS software package version 20.0. (IBM Corp., Armonk, NY, USA). The Kolmogorov-Smirnov, Shapiro, and D’Agostino tests were used to verify the normality of the distribution of variables and comparisons between groups. Categorical variables were assessed using the χ2 test (Fisher or Monte Carlo). ANOVA was used to compare the groups studied, followed by the post hoc test (Tukey) for pairwise comparison. The KruskalWallis test was used to compare different groups for abnormally distributed quantitative variables, followed by the post hoc test (Dunn’s multiple comparisons test) for pairwise comparison. Spearman’s coefficient was used to correlate between quantitative variables. The receiver operating characteristic curve (ROC) was used to determine the diagnostic performance of the markers. An area of more than 50% gives an acceptable performance, and an area of approximately 100% is the best performance for the test. The significance of the results obtained was judged at the 5% level.
Results
Clinical characteristics of non-Hodgkin lymphoma patients
Patients with early-stage NHL (group I) included 30 patients (60%) classified as stage I (Ann Arbor staging), while 20 patients (40%) had two or more involved sites of disease on the same side of the diaphragm and were classified as stage II. Group II (advanced stage) included 29 patients (58%) with involved sites on both sides of the diaphragm and were classified as stage III, while 21 patients (42%) had involved sites on both sides of the diaphragm with extranodal involvement and were classified as stage IV (Table 1).
Table 1
Ann Arbor staging of the studied patients
| Ann Arbor stage | Early (n = 50) | Advanced (n = 50) |
|---|---|---|
| I | 30 (60) | 0 (0) |
| II | 20 (40) | 0 (0) |
| III | 0 (0) | 29 (58) |
| IV | 0 (0) | 21 (42) |
Our study involved 100 patients diagnosed with B-cell NHL based on the WHO classification, which takes into consideration the histopathology of the disease.
Sixty-two patients were diagnosed with diffuse large B-cell lymphoma, which is considered the most common subtype of NHL.
Twenty-four patients with follicular lymphoma (the major subtype of indolent lymphoma) were included in the study, while seven patients were diagnosed with mucosa-associated lymphoid tissue (MALT) lymphoma.
Four patients with small lymphocytic lymphoma were included in the study, while mantle cell lymphoma (a subtype of B-cell NHL that mostly affects old age) was diagnosed in three patients (Table 2).
Biochemical parameters of patients with non-Hodgkin lymphoma
A significant decrease in haemoglobin levels was detected in group I (10.15 ±1.26) and group II patients (10.37 ±1.39) compared to those in the control group (12.93 ±1.52) (p < 0.001). There was a significant decrease in the number of platelets among patients in group I (204.3 ±43.6) and group II (222.42 ±85.34) compared to that in the control group (264.78 ±64.19) (p < 0.001). A significant increase in the number of total white blood cells (WBCs) was detected in group I (6.69 ±1.66) and group II patients (mean = 7.76 ±6.04) compared to that in the control group (5.25 ±1.19) (p = 0.003) (Table 3).
Table 3
Comparison between the three studied groups according to complete blood count (CBC)
[i] Group I – early, Group II – advanced, WBCs – white blood cells, F – ANOVA test, Pairwise comparison between each 2 groups was done using post hoc test (Tukey), p1 – p value for comparing between early and advanced, p2 – p value for comparing between early and control, p3 – p value for comparing between advanced and control, * statistically significant at p ≤ 0.05
Serum soluble TLR2 and serum soluble TLR4 levels
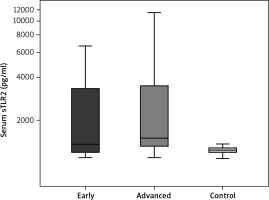
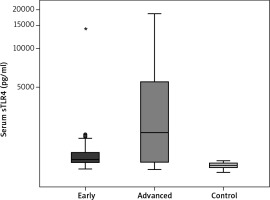
A significant increase in the serum level of sTLR2 (pg/ml) was detected among patients in group I (2381.1 ±1822.0) compared to that in the control group (1229.2 ±70.55) (p < 0.001). A significant increase in the serum level of STLR2 (2864.9 ±2599.9) was observed among group II patients compared to that in the control group (1229.2 ±70.55) (p < 0.001). Moreover, a significant increase in the serum level of sTLR2 was detected in group II (2864.9 ±2599.9) compared to that of group I (2381.1 ±1822.0) (p = 0.041) (Table 4, Fig. 1). Regarding the serum level of sTLR4, there was a significant increase in the serum levels of sTLR4 (pg/ml) among group I patients (2465.4 ±3501.8) compared to those in the control group (1242.3 ±53) (p < 0.001). A significant increase in the serum level of sTLR4 was also detected among patients in group II (4759.7 ±5176.2) compared to those in the control group (1242.3 ±53) (p < 0.001). However, no significant increase was detected in the serum levels of sTLR4 when comparing group II (4759.7 ±5176.2) to group I (2465.4 ±3501.8) (Table 5, Fig. 2).
Table 4
Comparison between the three studied groups according to serum level of soluble toll like receptor 2 (sTLR2)
[i] Group I – early, Group II – advanced, H – Kruskal-Wallis test, Pairwise comparison between each 2 groups was done using post hoc test (Dunn’s test for multiple comparisons), p1 – p value for comparing between early and advanced, p2 – p value for comparing between early and control, p3 – p value for comparing between advanced and control, * statistically significant at p ≤ 0.05
Table 5
Comparison between the three studied groups according to serum level of soluble toll like receptor 4 (sTLR4)
[i] Group I – early, Group II – advanced, H – Kruskal-Wallis test, Pairwise comparison between each 2 groups was done using post hoc test (Dunn’s for multiple comparisons test), p1 – p value for comparing between early and advanced, p2 – p value for comparing between early and control, p3 – p value for comparing between advanced and control, *statistically significant at p ≤ 0.05
Correlation between serum levels of sTLR2, sTLR4, and clinicopathological parameters
A significant positive correlation was detected between the serum levels of sTLR2 and disease staging according to the Ann Arbor staging (r = 0.490) (Table 6, Fig. 3).
Fig. 3
Correlation between serum sTLR2 (pg/ml) and Ann Arbor staging in non-Hodgkin lymphoma patients
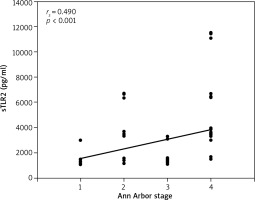
Table 6
Correlation between Ann Arbor staging and serum sTLR2 in non-Hodgkin lymphoma patients
A significant positive correlation between the serum levels of sTLR4 and the Ann Arbor staging of disease was also detected (r = 0.470) (Table 7, Fig. 4).
Fig. 4
Correlation between serum sTLR4 (pg/ml) and Ann Arbor staging in non-Hodgkin lymphoma patients
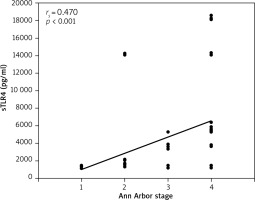
Table 7
Correlation between Ann Arbor staging and serum sTLR4 in non-Hodgkin lymphoma patients
Diagnostic performance of sTLR2
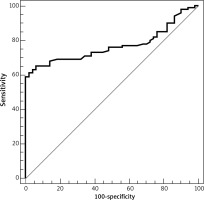
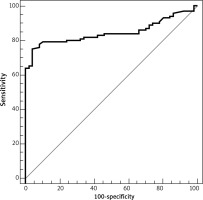
ROC analysis of sTLR2 revealed that the area under the curve was 0.769. At a cut-off value > 1314.5, the sensitivity and specificity of serum sTLR2 in the differentiation between healthy individuals and patients with NHL were 65% and 94%, respectively (Table 8, Fig. 5).
Diagnostic performance of sTLR4
The ROC analysis of serum sTLR4 revealed that the area under the curve was 0.845. At a cut-off value > 1305.5, the sensitivity and specificity of sTLR4 in the differentiation between healthy individuals and patients with NHL were 75% and 96%, respectively (Table 9, Fig. 6).
Discussion
TLRs are a family of pattern recognition receptors that play an important role in host defence against infection [10]. TLRs have been suggested to play a major role in carcinogenesis via activation of NK-κB and MAPKs, resulting in the production of cytokines and the maintenance of chronic inflammation [4, 11].
Several studies have reported that the cells of several tumours display increased expression of TLRs [5]. They are expressed in different types of cancers as tumour promoters [12]. Lymphocytes are immune cells that express a large number of TLRs; therefore, abnormal levels or signalling of TLRs may lead to the progression of malignant lymphomas [11]. TLR signalling must be tightly controlled in both physiologic and pathologic states to prevent subsequent damaging events that may occur due to either over- or sub-activation of the system [13]. Soluble toll-like receptors are considered the first-line negative regulatory mechanisms that act as strong competitors for TLR agonists that inhibit their binding with TLRs [6].
To the best of our knowledge, this study is the first to assess the serum levels of sTLR2 and sTLR4 in patients with NHL and to investigate their correlation with the clinicopathological parameters of these patients.
The present study revealed a significant increase in the serum levels of STLR2 and STLR4 in patients with NHL when compared to those in the control group. Moreover, by studying the correlation between sTLR2, sTLR4, and clinicopathological parameters, a significant positive correlation was found between serum levels of sTLR2 and sTLR4 and the Ann Arbor staging of NHL patients.
This was in agreement with the study conducted by Wei et al. [14], who found that the serum level of STLR4 was elevated among patients with non-small lung cancer and found that there was a positive correlation between STLR4 serum level and the stage of disease.
The significant elevation of serum sTLR4 agrees with the study conducted by Ten Oever et al. [15] in which the rapid elevation of sTLR4 in plasma upon lipopolysaccharide (LPS) administration as a proinflammatory cytokine showed the rapid activation of a feedback mechanism to counteract the activation of TLR4 signalling.
Soluble forms of TLR2 have been detected in human fluids such as breast milk, amniotic fluid, saliva [16, 17], and in synovial fluid of human joints with osteoarthritis [18]. STLR2 is generated from the transmembrane TLR2 via proteolytic cleavage of the extracellular domain of TLR2, resulting in the generation of at least six isomers [19]. STLR2 may act as a decoy receptor that binds to ligands recognised by TLR2 and thus inhibits TLR signalling, or it may interact with CD14, which is a TLR2 coreceptor and inhibits the TLR2-CD14 interaction, which is essential for TLR signalling [13].
On the other hand, the soluble forms of TLR4 that were found in saliva and in synovial fluid of osteoarthritic joints are produced by mechanisms (i.e., alternative splicing) other than post-translational modification [19].
STLR4 is suggested to form a complex with MD-2 and inhibit formation of the TLR4-MD-2 complex, which is needed for ligand binding and thus inhibits TLR signalling [15]. Moreover, sTLR4 may disrupt LPS signalling via TLR4 through interaction with CD14 and/or LPS binding protein, which are both cofactors that are essential for ligand binding with TLR4 [20].
Conclusions
In the present study, we investigated the sensitivity and specificity of sTLR2 and sTLR4 to differentiate between healthy individuals and patients with NHL using ROC curves. The sensitivity and specificity of sTLR4 in the differentiation between healthy individuals and patients with NHL were 75% and 96%, respectively, which were higher than those of sTLR2 (65% and 94%). In conclusion, serum STLR2 and STLR4 as endogenous negative regulators of TLR signalling might be considered as diagnostic and prognostic markers for NHL. Further studies are recommended to investigate the role of TLRs and their negative regulators in tumour biology.