Introduction
Technological improvements in drug-eluting stents (DES) have led to drastic reductions in restenosis and acute stent thrombosis rates. However, chronic inflammation and late and very late stent thrombosis, particularly with the first generation durable polymer DES (DP-DES), prompted the development of new DES platforms that use biodegradable polymers. The advantage of biodegradable polymers relies on the resorbable property of these polymers, reducing in this way the polymer-induced chronic inflammation and related clinical events such as restenosis and stent thrombosis. Notwithstanding , despite the theoretical advantages of bioabsorbable polymer DES (BP-DES), there are limited data focused on its very early vascular healing process.
Aim
Therefore, we aimed to assess the 1-month vascular healing response following implantation of the bioabsorbable polymer-coated sirolimus-eluting stent Alex Plus (BP-SES, Alex Plus, Balton, Warsaw, Poland).
Material and methods
Study population
In this prospective, single-centre study, patients aged 18 years and older undergoing percutaneous coronary intervention (PCI) for treatment of de novo coronary lesions with implantation of a bioresorbable polymer sirolimus-eluting stent (BP-SES), Alex Plus, for any clinical indication were enrolled. Major exclusive criteria were renal failure (glomerular filtration rate less than 45 ml/min/1.73 m2), allergy to contrast media or any of the stent components, hemodynamic compromise at presentation, pregnancy as well as participation in other clinical trials.
Stent system description
Alex Plus is composed of a laser-cut cobalt-chromium alloy stent platform with open-cell geometry and 71 μm struts with a fully biodegradable circumferential coating (copolymer of poly-L-lactic and glycolic acid) and the anti-proliferative drug sirolimus. Experimental studies showed 95% release of the drug after 28 days and practically full polymer biodegradation after 8 weeks following Alex Plus implantation [1].
Optical coherence tomography
Optimal coherence tomography (OCT) using iLumien OPTIS Medical system (Abbott Vascular, Santa Clara, CA, USA) imaging was performed before and after stent implantation as well as at 1-month follow-up. The OCT registration included the entire stented segment as well as 5 mm proximally and distally to the stent. The OCT image was acquired using automated pullback triggered by the manual injection of contrast. The Review Workstation (Abbott, Santa Clara, CA, USA) was used for offline analysis of the treated segment. The region of interest was selected between the proximal and distal edges of the stent. The analysis was performed every 1 mm to measure lumen area (LA), lumen diameter (LD), endoluminal stent area (SA), minimal stent diameter, % area stenosis (%AS) and strut apposition to the vessel wall after implantation. Malapposition was defined as a visible space between the virtually drawn strut and the lumen contour or if the distance between the strut blooming and lumen counter was more than 71 μm. Furthermore, struts’ coverage was also assessed at 1-month follow-up.
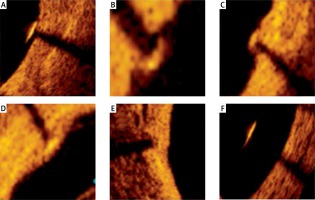
Based on OCT, the early coverage of each stent strut was classified using a previously published classification: definitely uncovered, uncovered fibrin, partially covered, covered protruding, covered embedded, malapposed (Figure 1). A definitely uncovered strut was defined as a strut not covered at any side. A partially covered strut was defined as a strut partially covered by tissue but only at one side. A covered protruding strut was defined as a strut covered by tissue on both sides but extending into the lumen. A covered embedded strut was defined as a strut covered by tissue or neointima and not extending into the lumen contour. An uncovered fibrin strut was defined as an uncovered strut covered with fibrin. A malapposed strut was present if the distance between the stent blooming artefact to the endoluminal surface was greater than the thickness of the strut equal of 71 μm. If the strut was covered with tissue, the thickness was measured as the distance between the blooming artefact and endoluminal surface [2].
Figure 1
Optical coherence tomography-based stent strut coverage classification: definitely uncovered (A), uncovered fibrin (B), partially covered (C), covered protruding (D), covered embedded (E) and malapposed (F)
The neointimal area was measured by subtracting as follows: endoluminal SA – (LA + malapposition area).
Statistical analysis
The Shapiro-Wilk test was used to analyse the continuous data distribution. Normally distributed values were presented as the mean ± standard deviation. Non-normally distributed values were presented as the median with 25th and 75th percentile (interquartile range – IQR). Categorical variables were summarized using percentages and counts. Statistica (Statistica v. 13, Tibco Software Inc. Palo Alto, USA) was used for statistical analysis.
Results
Study population
Twelve patients underwent PCI with implantation of one Alex Plus per patient in de novo lesions in native coronary arteries. The mean patient’s age was 71 years; 66.7% of them were males. Seventy-five percent of patients had chronic coronary syndrome (CCS), 25% of patients had acute coronary syndrome (ACS), and 17% had diabetes mellitus type 2. All patients were treated with dual antiplatelet therapy (DAPT) during the entire duration of the study. One month of OCT imaging was performed in all patients.
OCT analysis
Stent analysis
The mean lesion length was 24.6 ±6.6 mm. Before stent implantation, minimal LA and minimal LD were respectively 2.3 ±2.2mm2 and 1.51 ±0.6 mm. The mean length of the implanted stents was 24.9 ±7.34 mm. In a 1-month follow-up, the minimal LA and minimal LD were 5.98 ±2.73 mm2 and 2.58 ±0.58 mm respectively.
Cross-section analysis
In the follow-up, 2021 stent struts from 269 cross-sections were assessed (Figure 2). The mean neointimal thickness at covered struts was 12 μm and the mean neointimal area was 0.62 mm2. The percent area stenosis at follow-up was 8.5 ±6.7%. A total of 63 (3.12%) stent struts were malapposed and mean malapposition area was 0.51 ±0.34 mm2. Furthermore, 67 (3.3%) of all struts were defined as definitely uncovered, and in 9 stents, at least one strut was defined as definitely uncovered. Additionally, 1788 (88.5%) struts had evidence of coverage (classified either as covered embedded or covered protruding). Stent analysis at 1-month follow-up is summarised in Table I.
Table I
OCT analysis at baseline and 1-month follow-up
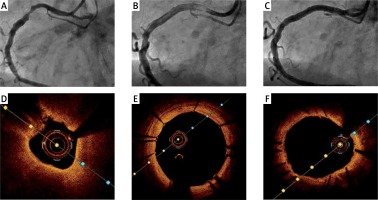
Figure 2
Representative optical computed tomography (OCT) imaging before and after Alex Plus implantation as well as at 1-month follow-up. A – Baseline angiography of a patient with stenosis of the right coronary artery (RCA). B – Implantation of Alex Plus stent. C – Angiography at the 1-month follow-up. D – OCT cross-sectional image before Alex Plus implantation. E – OCT cross-sectional image after Alex Plus implantation. F – OCT cross-sectional image at the 1-month follow-up
Discussion
The major finding of this study is that the Alex Plus has a favourable coverage rate at 1 month.
First generation DP-DES significantly improved clinical outcomes compared to bare-metal stent (BMS); however, late and very late stent thrombosis following DP-DES implantation was associated with polymer-induced chronic inflammation and hypersensitivity reactions [3]. Therefore, in the concept of using polymers which would fully degrade after completion of drug elution, BP-DES were designed to reduce those adverse effects of first generation DP-DES. The biodegradable polymer is designed to fully degrade from the strut surface after the elution of the anti-proliferative drug is completed. It was previously reported that the copolymer of poly-L-lactic and glycolic acid covering the assessed Alex Plus undergoes full bioresorption after 8 weeks [1]. Furthermore, the stent strut thickness was demonstrated to play an important role in the late stent thrombosis mechanism [4]. Additionally, previous studies showed that quicker and proper neointimal coverage might play a key role in shortening DAPT [5]. It was previously found that another cobalt-chromium BP-SES (BuMa Supreme, SINOMED, Tianjin, China) exhibited a higher rate of covered struts at 1-month follow-up as compared to cobalt-chromium durable polymer everolimus-eluting stent (DP-EES, XIENCE stent, Abbott Vascular, Santa Clara, CA, USA) [6]. In our study, the composition of the biodegradable coating and fast drug release kinetics, as well as low strut thickness of Alex Plus, resulted in a favourable healing profile expressed by the relatively high rate (88.5%) of covered struts (embedded covered and protruding covered struts). The results of our study, which showed 11.5% of struts without evidence of complete coverage, are favourable as compared to the previously published 1-month coverage for another cobalt-chromium BP-SES (Orsiro DES, Biotronik AG, Bulach, Switzerland) which demonstrated 19.6% uncovered struts analysed in patients with ST elevation myocardial infarction [7]. However, it needs to be stressed that the higher rate of the uncovered struts might be attributed to the exclusively ST-elevation myocardial infarction (STEMI) population.
Despite the reduction of the chronic inflammation process, late stent thrombosis still occurs after implantation of BP-DES. Pathological studies showed that late stent thrombosis might also be a result of uncovered and malapposed struts’ presence [8]. As compared to DP-ZES, we observed a numerically lower percentage of malapposed and uncovered struts (BP-SES 5.7% vs. DP-ZES3.12% and BP-SES 12.3% vs. DP-ZES 4.6% respectively) [9]. Additionally, our results are comparable to EGO-BIOFREEDOM results of 1-month follow-up after implantation of thick-strut (112 μm) polymer-free DES (85.8% of struts defined as covered) [10]. Furthermore, results of a large randomized study demonstrated that among patients at high bleeding risk, the 1-month DAPT after DP-ZES implantation therapy was non-inferior to a polymer-free DES with regard to death from cardiac causes, myocardial infarction or stent thrombosis [11, 12]. In summary, our study demonstrated comparable strut coverage and a favourable healing profile when compared to the previous study regarding 1-month healing response after DP-ZES implantation [9]. It may suggest that the evaluated Alex Plus might be considered as a potential candidate for short DAPT therapy in the future. However, a large randomized clinical trial is needed for verification of the results of this study.
There are several study limitations that have to be mentioned. First, the small population of the study might bias the final results. The study assessed only the sirolimus stent platform and did not provide data for a control group. Moreover, OCT does not distinguish healthy from abnormal tissue, which may overestimate the final assessment of the stent healing process.







