Summary
Both self-expanding and balloon-expandable prostheses are used in transcatheter aortic valve implantation (TAVI). Many studies assessing the early safety of TAVI procedures depending on the type of valve prostheses (balloon-expandable and self-expandable) concern femoral access. To our knowledge, there are no studies evaluating the impact of the type of prosthesis on the safety and efficacy of transcarotid TAVI. However, it is known that the access through the common carotid artery may be an alternative for patients in whom femoral access is not available.
Introduction
Transcatheter aortic valve implantation (TAVI) has become a standard of care for patients with severe symptomatic aortic stenosis (AS) who are not eligible for conventional surgical aortic valve replacement (SAVR) and have high surgical risk. Also, based on the heart team decision it is an accepted treatment for intermediate and low-risk patients with additional factors favoring the non-surgical approach [1–3]. The transfemoral (TF) access is preferred in all patients because it has the lowest complication rate [4, 5]. However, in 15–20% of TAVI patients TF is impossible due to concomitant peripheral arterial diseases (PAD) and abnormalities of the thoracic and abdominal aorta, increasing the risk of bleeding and vascular complications [6–8]. Since the first TAVI via the left common carotid artery (CCA) described by Modine et al. in 2010 [9] transcarotid (TC) access has been used as an alternative for patients disqualified from TF-TAVI [10, 11]. Both balloon-expandable (BE) and self-expandable (SE) valves can be implanted through the CCA; however, no direct comparisons have been published [10–12].
Aim
The purpose of our registry including all consecutive patients treated with TC-TAVI using BE and SE valves in a large academic institution was to evaluate early safety and efficacy of the procedure depending on the type of implanted valve.
Material and methods
The study group consisted of patients with severe symptomatic aortic stenosis who underwent the TC-TAVI procedure in our hospital between 2017 and 2020. Since we aimed to investigate outcomes in the TC-TAVI procedure, patients with only balloon aortic valvuloplasty were excluded from the final analysis. All patients signed written informed consent to the proposed treatment. Because of the retrospective study design, no institutional review board permission was required. The results were defined and presented according to the Valve Academic Research Consortium-2 (VARC-2) consensus [13]. The decision on qualification for the TC-TAVI procedure and selection of the size and type of implanted valve prosthesis, BE or SE, was made by the multidisciplinary Heart Team. The access and sizing were planned based on the multislice computed tomography (MSCT) results of the peripheral arteries and aorta, coronary angiography, and transthoracic echocardiography (TTE). MSCT analysis was performed using the 3Mensio (Pie Medical Imaging, The Netherlands) software. The presence of thoracic and abdominal aorta disease (aneurysm, thrombus, chronic dissection, history of a stent graft, tortuosity) as well as peripheral artery disease (PAD), small diameter (< 6 mm), severe calcifications and severe angulation of the iliofemoral arteries disqualified the patient from the femoral access. The MSCT analysis method for carotid access and the operating technique has been described in detail in the previous article [14]. Patients who had CCA diameter more than 5.5 mm and no significant stenosis (> 50%), excessive angulation, and calcifications were qualified for TC-TAVI. All procedures were performed in the hybrid operating room during general or local anesthesia by the multidisciplinary TAVI team. Every patient received a pre-procedural intravenous dose of antibiotic (1.5 g cephazolin) to prevent infective endocarditis. Heparin was given in a dose of 100 U/kg (activated clotting time > 250 s). After placement of two 5-0 monofilament continuous purse-string sutures, the 6-Fr vascular sheath was inserted into the carotid artery. The pigtail catheter was positioned in the noncoronary sinus using a 6-Fr sheath inserted through the radial or femoral artery. An endocavitary electrode for rapid ventricular pacing was introduced into the right ventricle through the femoral or jugular vein. Intraoperative monitoring of each patient included regional cerebral oximetry (Covidien, Medtronic plc, Ireland), arterial blood pressure, central venous pressure, saturation of arterial blood, electrocardiography, and transthoracic (TTE) or transoesophageal echocardiography (TOE). Selective carotid angiography was performed to assess arterial patency after the removal of the delivery system.
Statistical analysis
Categorical data were presented as numbers (%). The Kolmogorov-Smirnov test was used to assess the data distribution. Normally distributed values were presented as mean with standard deviation. Non-normally distributed values were presented as median with 25th and 75th percentile (interquartile range (IQR)). Continuous data were compared by Student’s t-test or by the Mann-Whitney U test, depending on the distribution. Categorical data were analyzed with the χ2 or Fisher’s exact test. P-values of < 0.05 were considered statistically significant. The statistical analysis was performed using MedCalc 17.9.2 (MedCalc software).
Results
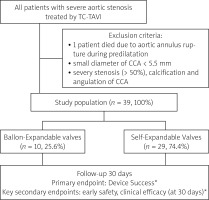
Forty patients were qualified for the TC-TAVI procedure from September 2017 to October 2020 in the Upper Silesian Medical Center of the Medical University of Silesia in Katowice, Poland. One patient was excluded from the analysis because he died intraoperatively prior to aortic valve implantation. The cause of death was a rupture of the aortic annulus during predilation. The retrospective study finally enrolled 39 patients (10 (25.6%) BE-TAVI and 29 (74.4%) SE-TAVI) (Figure 1). The baseline characteristics of the patients are included in Table I. The groups did not differ in age (74.5 (69–81) vs. 77 (72–84.2); p = 0.349) or gender (80% males vs. 44.8%; p = 0.073). All patients were symptomatic with baseline NYHA class ≥ III. Patients in SE-TAVI had a higher incidence of chronic obstructive pulmonary disease (COPD) (34.5% vs. 0%; p = 0.040). The groups did not differ significantly in terms of other comorbidities although the calculated risk of mortality according to the EuroSCORE was higher in the BE-TAVI group (10.8% (6.2–14.0) vs. 5.5% (4.3–8.7); p = 0.027). The preoperative blood test results (level of creatinine, glomerular filtration rate (GFR), hemoglobin, hematocrit, white blood cells (WBC), platelets (PLT)) were comparable. Also, echocardiographic parameters (left ventricular ejection fraction (LVEF); aortic valve maximal gradient (PG max); aortic valve mean gradient (PG mean); aortic valve area (AVA); transaortic peak instantaneous velocity (Vmax); mitral regurgitation (MR) > 2 grade) and MSCT parameters (diameter left and right CCA, the occurrence of bicuspid aortic valves (BAV); height of left main coronary artery (LM) and right coronary artery (RCA); perimetry; aortic valve annulus area (AVAA)) were comparable between groups. The following valve types were used: Edwards-Sapien 3 Ultra (Edwards Lifesciences Corp. Irvine, CA, USA) in the BE-TAVI group; Evolut R (Medtronic, Minneapolis, MN, USA) and Portico (Abbott Vascular, Santa Clara, CA, USA) in the SE-TAVI group. The prostheses’ characteristics are presented in Table II. The size of the implanted BE valves was smaller than the SE valves (26.0 (23.0–26.0) vs. 29.0 (26.0–29.0); p < 0.001). All balloon-expandable valves were implanted through a dedicated vascular sheath, while in the self-expandable valves group, the vascular sheath was used only in 7 patients. Perioperative and postoperative results are shown in Table III. 100% device success was observed in both groups. There were no procedural deaths in patients in whom the valve was implanted. All of BE-TAVI and 93.1% of SE-TAVI were performed under general anesthesia (p = 1.000). Coronary occlusion, prosthetic dislocations and dysfunction which required conversion to SAVR were not observed in either group. There was a similar number of other events defined by VARC-2: myocardial infarction, major vascular complication, life-threatening bleeding, and acute renal failure in BE and SE groups (Table III). Most of the procedures were performed through the LCCA and a minority through the RCCA (9/10 BE and 28/29 SE). The procedural time, as well as the percentage of balloon aortic pre- and postdilatation (10% vs. 6.9%; p = 1.000 and 10% vs. 20.7%; p = 0.652 respectively), was not significantly different between the groups. A low rate of neurological complications was observed in both the BE-TAVI and SE-TAVI group (no strokes and only 1 TIA (10%) vs. 2 (6.9%); p = 0.753, respectively) (Table III). These data confirm cerebral oximetry results bilaterally during the procedure (Table IV, Figure 1). The time patients spent in the ICU was similar, while the total hospitalization time was shorter in the BE-TAVI group (5.8 ±0.6 days vs. 6.4 ±0.9 days; p = 0.043). There was no statistically significant difference in the percentage of atrial fibrillation (0% vs. 6.9%; p = 1.000) or pacemaker implantation (10% vs. 13.8%; p = 1.000). Comparison of the postoperative echocardiography parameters (EF, PG max, PG mean) and the NYHA functional class showed no significant differences between the groups. (Table II). Regardless of the types of prostheses, we noted a similar reduction of AV gradient (PG max and PG mean) from 68.0 (60.2–87.5) to 15.0 (13.0–20.0); p < 0.001 and from 41.0 (35.2–50.2) to 8.0 (6.2–10.0); p < 0.001 respectively, with no effect on EF (50.0 (45.0–55.0) vs. 55.0 (50.0–60.0); p = 0.318). Also there was an improvement of NYHA class at follow-up (3 (3–3) vs. 2 (1–2); p < 0.001). The type of the valve prosthesis did not affect 30-day mortality (0 (0%) vs. 2 (6.9%); p = 1.000, respectively) in the BE and SE group. One patient died of an unknown cause 2 weeks after discharge, while the second died 3 weeks after discharge from the hospital due to complications after COVID-19 infection.
Table I
Baseline clinical characteristics of patients
[i] Data are given as the median (interquartile range) or as n (%). AVA – aortic valve area, AVAA – aortic valve annulus area, BAV – bicuspid aortic valve, BE – balloon-expandable, BMI – body mass index, COPD – chronic obstructive pulmonary disease, GFR – glomerular filtration rate, LCCA – left common carotid artery, LM – left main coronary artery, LVEF – left ventricular ejection fraction, MI – myocardial infarction, MR - mitral regurgitation, MSCT – multislice computed tomography, NYHA – New York Heart Association, PAD – peripheral arterial disease, PCI – percutaneous coronary intervention, PG max – aortic valve maximal gradient, PG mean – aortic valve mean gradient, PLT – platelets, RCA – right coronary artery, RCCA – right common carotid artery, SE – self-expandable, TAVI – transcatheter aortic valve implantation, TNT – troponin T, Vmax – transaortic peak instantaneous velocity, WBC – white blood cells.
Table II
Valves’ characteristics
[i] BE – balloon-expandable, SE – self-expandable, TAVI – transcatheter aortic valve implantation. The size of the vascular sheath on a French scale. Edwards-Sapien 3 Ultra (Edwards Lifesciences Corp., Irvine, CA, USA), Evolute R (Medtronic, Minneapolis, MN, USA), Portico (Abbott Vascular, Santa Clara, CA, USA).
Table III
Periprocedural and postprocedural outcomes
[i] Data are given as the median (interquartile range), as the mean (standard deviation) or as n (%). BE – balloon-expandable, GFR – glomerular filtration rate, ICU – intensive care unit, LCCA – left carotid common artery, LVEF – left ventricular ejection fraction, Pg max – aortic valve maximal gradient, Pg mean – aortic valve mean gradient, PLT – platelets, PVL – paravalvular leak, SAVR – surgical aortic valve replacement, SE – self-expandable, TAVR – transcatheter aortic valve replacement, TIA – transient ischemic attack, TNT – troponin T, Vmax – transaortic peak instantaneous velocity, WBC – white blood cells.
Table IV
Results of cerebral oximetry during procedure
Figure 1
Schedule of the study groups
TC-TAVI – transcarotid transcatheter aortic valve implantation, CCA – common carotid artery. Balloon-explandable valve: Edwards-Sapien 3 Ultra (Edwards Lifesciences Corp. Irvine, CA, USA), Self-explandable valve: Evolute R (Medtronic, Minneapolis, MN, USA) and Portico (Abbott Vascular, Santa Clara, CA, USA). *According to the Valve Academic Research Consortium-2 (VARC-2) consensus.
Discussion
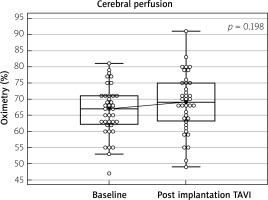
TAVI through the femoral artery is the preferred access most often used due to the safety [15]. Despite the reduction of the size of access sheaths and the modification of the vascular closure devices (Proglide, Manta), not all patients can undergo TF-TAVI and need alternative access sites. In many centers, TC access has become the first choice for patients for whom the femoral approach is not available. In our hospital, the TC-TAVI percentage is approximately 10% of all TAVI procedures, and it is similar to the result reported by other authors [16, 17]. The common carotid artery is easy to access because of its superficial location and size. The carotid artery preparation technique is not complicated but requires special attention to protect the jugular vein, the vagus, and the laryngeal nerves. The most common local access complications are injury to the vagus nerve, local hematoma, and wound infection [18]. In our group, of these complications, we observed only local hematoma in 1 patient in the BE group. Cerebrovascular events related to the use of the CCA approach, including stroke and TIA, are estimated at 2–4% [11, 19, 20]. In our registry, there was no stroke, and we observed 3 cases of TIA (7.7% in 39 patients), which were not related to the type of prosthesis and resolved within 4 h. We believe that the two aspects are very important in preventing severe neurological complications. The first is a detailed analysis of the MSCT during qualification, allowing one to choose an artery with a larger diameter, less calcification, and tortuosity, and with a more favorable spatial relationship between the virtual CCA centerline and the plane of the aortic annulus. It has a significant impact on facilitating and shortening the time of the procedure. Confirmation in the MSCT of the borderline dimensions of CCA allows one to consider using a sheathless technique for the planned SE valve. The second aspect is the continuous monitoring of cerebral oximetry throughout the procedure, which allows for a quick response to possible brain perfusion disorders. In a meta-analysis of Wee et al. [19] (3.8% neurological complication rate in 364 patients) cerebral oximetry was the main tool for periprocedural cerebral monitoring. Our analysis did not reveal any differences between groups in left and right side cerebral oximetry values in the successive steps of the procedure (Table IV). In addition, in the comparison of oximetry results for all TC-TAVI, the baseline values did not differ from the values after implantation (Figure 2). In our center, during the TC-TAVI procedure, we do not perform a 2–3 min clamping of the carotid artery to check for the Willis circle function, as proposed by some operators [10, 12]. In our surgical technique, the carotid artery was carefully dissected, especially protecting the vagus nerve, and manually examined after dissection for the presence of calcifications. Two 5-0 monofilament continuous purse-string sutures were used similarly to the technique used to cannulate the ascending aorta during the classical surgical operation. After the procedure, the carotid artery was closed using sutures without clamping. We do not prefer clamping the carotid artery to reduce its trauma. In cerebral oximetry, we did not observe a difference in saturation between the left and right sides after leading the delivery sheath (14, 16 Fr) (oximetry values were not lower than 55%); this allowed the procedure to be continued safely. In our observation, valve selection had no effect on the other endpoints according to the VARC-2 consensus. Percentages of life-threatening bleeding (2.6%), myocardial infarctions (2.6%), major vascular complications (2.6%), acute kidney injuries (0%), pacemaker implantations (12.8%), and all mortality (6.9%) for all the TC-TAVI group were comparable with the results reported by other authors. Similar results were obtained for the choice of implantation site (LCCA-94.9% (90% in the BE group and 96.6% in the SE group)) and type of anesthesia (general anesthesia – 94.9% of all TC-TAVI (100% in the BE group and 93.1% in the SE group)) [11, 16, 17, 19]. We prefer general anesthesia with intubation because this method increases security by reducing the uncontrolled movement of the patient. However, in special situations, we successfully performed TC-TAVI using SE valves in 2 patients. The results of large analyses comparing the implantation of two types of valves (BE vs. SE) implanted transfemorally are inconclusive. In the French registry comparing the results of the Sapien 3 BE valve with the Evolut R SE valve, 20,918 patients showed lower numbers of all-cause death, cardiovascular death, rehospitalization for heart failure, and pacemaker implantation in the group of patients with a Sapien 3 BE valve [21]. On the other hand, Vlastra et al., analyzing 12 381 patients, did not confirm lower 30-day mortality in the BE valve group. They found lower incidence of strokes and pacemaker implantation but more life-threatening bleeding events in these groups [22]. In our analysis, all perioperative and postoperative outcomes in the study groups were comparable. Independent of valve prosthesis type, significant reduction of NYHA class, and improvement of echocardiographic parameters (Pg max, Pg mean, Vmax, and low number of severe (> 2 grade) paravalvular leaks (PVL)) were observed. These data confirmed the good hemodynamic profile of the used prostheses, as well as the adequate size and correct implantation. Paravalvular leak, structural valve deterioration, prosthesis–patient mismatch, leaflet thrombosis, and endocarditis can lead to faster valve dysfunction [23]. Morphometric analysis of aortic valve calcifications in MSCT described by Ryś et al. seems to be an interesting method to reduce the potential risk of PVL in TAVI procedures [24]. The choice between an SE or BE valvular prosthesis depends mainly on the analysis of MSCT (values of the aortic valve annulus area and annulus perimeter, height of the left and right coronary artery, and diameter of the common carotid arteries), type of the native valve (tricuspid vs. bicuspid), presence of the failed aortic bioprosthetic valve (ViV) and extent of calcifications (especially massive calcifications extending into the left ventricular outflow tract). In addition, in our opinion, in patients who have a smaller size of the common carotid arteries, it is worth using a self-expanding valve because it can be implanted without a dedicated vascular sheath. This may be important for blood flow through the carotid artery and the reduction of neurological complications. In patients with a higher EuroSCORE, we prefer to use BE valve prosthesis implantation because, in our opinion, this system is more predictable, avoids pre- and post-dilatation, and the procedure time is shorter, which may be important for the course and safety of the procedure.
Figure 2
Comparison baseline and post implanation results of the cerebral oximetry all trancarotid TAVI patient (n = 39)
TAVI – transcatheter aortic valve implantation
The presented analysis has several limitations. The study was retrospective, non-randomized, and single-center. Only the early results were assessed. Patients in the BE valve group had a higher EuroSCORE II risk. On the other hand, all of the TC procedures were performed consistently according to the institutional standard operating procedures. Importantly, the TC TAVI was always done under brain oxygenation monitoring.
Conclusions
Based on our registry, we can conclude that transcarotid access is safe and effective, independently of which types of valve prosthesis (balloon-expandable or self-expandable) were used. In our opinion, precise preprocedural multivariate analysis of MSCT and continuous monitoring of cerebral oximetry are key to the success of transcarotid TAVI.







