Summary
Silent cranial embolism due to carotid artery stenting (CAS) has been demonstrated to cause dementia and even ischemic stroke. The rate of periprocedural ipsilateral asymptomatic cranial embolism detected on cranial diffusion-weighted magnetic resonance imaging was lower in the CAS procedures in which optimal predilatation was performed but postdilatation after stent deployment was not performed compared to the CAS procedures in which suboptimal predilatation and postdilatation after stent deployment were performed.
Introduction
With the development of interventional devices and techniques, carotid artery stenting (CAS) has become an alternative to carotid endarterectomy (CEA) for the treatment of carotid artery stenosis [1]. The most important complications of carotid artery stenting include new ischemic cerebral lesions associated with distal embolization and neurological symptoms [2]. Silent cranial embolism associated with CAS has been demonstrated to cause dementia, cognitive decline [3] and even ischemic stroke [4].
Diffusion-weighted magnetic resonance imaging (DW-MRI) is a very sensitive method to detect silent cranial lesions that develop during CAS cranial DW-MRI [5, 6]. The use of embolic protection devices (EPD) in CAS has reduced the incidence of new cranial ischemic lesions associated with the procedure and detected through DW-MRI [7]. Therefore, EPD are strongly recommended to be used during CAS procedures [8].
Even though the CAS procedure is performed with the use of EPD, cranial embolism may yet develop. New ischemic cerebral lesions caused by distal emboli that occur during CAS may develop due to several factors. Some of these factors include clinical status of patients, their vasculature, aortic arch type, devices used (balloon, stent, catheter…), experience of operators, plaque morphology, etc. [9]. Hence patients, lesions and appropriate material selection play an important role to decrease distal emboli associated with CAS.
Transcranial Doppler (TCD) studies have shown that most CAS-associated periprocedural embolisms occur during the advancement of the wire, ballooning and stent deployment [10]. The procedural steps of CAS are still not clearly defined. In classical CAS, after the guidewire is advanced through the stenosis with a protection device, suboptimal predilatation (8–10 atmospheres (atm)) is performed with a small balloon (≤ 4 mm), the stent is deployed and balloon postdilatation (if residue is > 30%, with a 5.0–5.5 mm balloon at 8–10 atm)) is performed. If the lesion is > 95% and the distal embolic protection device cannot cross, ballooning is performed for the lesion without an embolic protection device (unprotected postdilatation). All the abovementioned procedural steps, especially balloon postdilatation, pose a risk for cranial embolism [11, 12]. There are studies showing that CAS procedures performed with suboptimal pre-stent ballooning but without poststent ballooning are safe and associated with better clinical outcomes [13].
The non-classical CAS method we use for certain patients is defined as follows: To avoid the risk of embolism especially due to unprotected predilatation, we used the proximal blockage system as the EPD (Mo.MA) for this group of patients. In this way, no unprotected predilatation was performed. Even though it was under EPD, we performed optimal predilatation (10–14 atm) with a larger balloon (≥ 4 mm) instead of suboptimal predilatation with a smaller balloon (≤ 4 mm). As we performed predilatation optimally, the stent can settle on the wall of the carotid artery wall and the residue stenosis is usually < 30%; therefore, there is no need for balloon postdilatation. In summary, the patients were divided into two groups: the classical group in which the stent was deployed after suboptimal predilatation and postdilatation after stent deployment was performed and the non-classical group in which the stent was deployed after optimal predilatation and postdilatation after stent deployment was not performed.
Aim
The purpose of this study was to compare the periprocedural asymptomatic cranial embolism rates of the classical CAS method and non-classical CAS method using cranial DW-MRI.
Material and methods
Method
We obtained the approval of the ethics board of our facility for this study (No: 2020-320). We included 367 clinically uncomplicated patients (mean age: 69.3 ±11.9) who were admitted to our center in the period from December 2010 to June 2020 and for whom CAS was decided after consultation in the multidisciplinary carotid council consisting of neurology, cardiology, cardiovascular surgery and radiology clinics. The patients were divided into 2 groups: those who underwent classical CAS with balloon postdilatation (130 patients) and those who underwent non-classical CAS without balloon postdilatation (237 patients). A symptomatic patient was defined as one who had a history of an ischemic cerebrovascular event with or without sequelae, transient ischemic attack (TIA), or amaurosis fugax in the previous six months. Patients who were symptomatic and had more than 50% stenosis on digital subtraction angiography (DSA) according to North American Symptomatic Carotid Endarterectomy Trial (NASCET) formulation, and those who were asymptomatic and had more than 80% stenosis were included in the assessment. All patients who had a glomerular filtration rate (GFR) greater than 60 ml/min/1.73 m² underwent computed tomography angiography (CTA) for the carotid after carotid Doppler ultrasonography (CDUS). Medical follow-up, CAS or CEA was decided by the multidisciplinary team depending on the clinical features, comorbidities and characteristics of carotid artery lesions of the patients. In our study, in order to clearly determine the risk of the CAS procedure completed without postdilatation causing cranial embolism, we determined several exclusion criteria such as haemodynamic instability during the procedure (> 10 min), difficult (type III aortic arch) and risky arcus aorta, severely tortuous carotid arteries, severely ulcerated, heavily thrombotic and heavily calcified circular carotid artery plaques (Gray-Weale type IV), watershed infarcts, history of unprotected CAS, and history of repeated ballooning.
Preparation of patients for carotid artery stenting
Patients were informed about the details of CAS and signed informed consent forms. Antihypertensive, antihyperlipidemic and antiplatelet medications that the patients had been taking were regulated. The procedure was initiated after their blood pressure values were regulated down below 135/80 mm Hg. We made sure that the patients had been taking dual antiplatelet therapy consisting of especially 100 mg of acetylsalicylic acid (ASA) and 75 mg of clopidogrel for at least 7 days. Otherwise, an additional loading dose (ASA 300 mg, clopidogrel 600 mg) and maintenance antiplatelet therapy were planned. A resistance test was performed on venous blood for both antiplatelet agents in the morning of the procedure. CAS was performed after a loading dose of 2 tablets of 90 mg ticagrelor and the 2x1 maintenance regimen if they had resistance only to clopidogrel.
Carotid artery stenting procedure
All procedures were performed by 2 operators with one being an invasive cardiologist and the other being an interventional vascular neurologist. They were performed under local anesthesia with percutaneous transfemoral access. The patient’s oxygen saturation, electrocardiographic and blood pressure parameters were monitored throughout the procedure. The procedure was initiated with a femoral 8 French (F) sheath. A 9F sheath was used when proximal protection was preferred as an embolic protection method. After the sheath was placed, all patients were given 75 IU/kg unfractionated heparin. Depending on the arcus aorta type of the patient evaluated in the council, a 5F hydrophilic head hunter or sım 1,2 diagnostic catheter was used. CAS was performed with the anchor method in most of the patients. The telescopic method was used in only a very few patients. Following bilateral carotid and cerebral DSA, we determined the embolic protection method to be used, balloon and stent diameters and whether predilatation/postdilatation would be performed. The stent design was not selected according to either the lesion or the vascular structure. The stent design that was available and actively used was then placed in the stenotic carotid artery. For predilatation, 3.0–5.0 × 20 mm balloons (Invader; Alvimedica, Simpass; Simeks) were used. For postdilatation, 5.0 –5.5 × 20 mm balloons (Viatrac; Guidant) were preferred. The balloon diameter for predilatation was calculated as around 1 mm smaller than the diameter of the distal intact ICA. Tapered stents were used for all patients. Self expandable stent diameter was adjusted as 20% larger than the diameter of the carotid artery measured digitally. The stent designs used at our clinic so far are: closed-cell stent; Xact carotid stent (Abbott), open-cell stents; Sinnus-carotid-conical RX stent (Optimed), RX Acculink stent (Abbott), Protege RX stent; Ev3, hybrid-cell stent; Cristallo ideale SE stent (Invatec). In the case of symptomatic and > 90% carotid artery stenosis, if the collateral carotid artery was not occluded totally, the collateral circulation in the carotid artery for which the procedure was planned was not poor on cerebral DSA, ICAs after bulbous area are tortuous, lesion was ulcerated and slightly thrombotic, proximal blockage system was preferred as EPD (Mo.MA). For the other lesions, the distal protection method (filter (Emboshield, Filterwire, spider FX)) was used. Patients with a heart rate of < 60/min were administered 1 mg of atropine intravenously (IV) before carotid ballooning. Atropine was given to the other patients if their heart rate went below < 60/min after ballooning/stenting. To make sure if there had been distal embolization associated with CAS, bilateral cerebral DSA was performed and compared with pre-CAS scans. For all patients who did not undergo coronary artery angiography (CAG) beforehand, CAG was performed after CAS.
Follow-up after carotid artery stenting
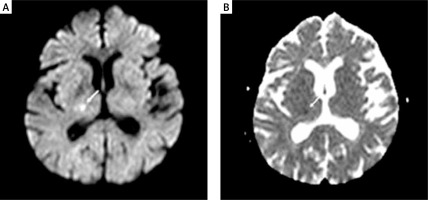
All patients were followed up for hemodynamic and clinical parameters at the coronary intensive care unit for 24 h following CAS. Cranial DW-MRI was performed to be able to see possible asymptomatic cranial microembolisms in patients 3–7 days before and 12–24 h after the CAS procedure (Figure 1). A routine cardiac enzyme test was not performed. The patients were followed up by the vascular neurologist for minor and major neurological complications for 24 h following the procedure. On discharge, all patients were prescribed dual antiplatelet and statin therapy (if low density lipoprotein (LDL) was > 70 mg/dl). Dual antiplatelet therapy was continued for 6–12 months if the patients did not have any other specific conditions. All patients were followed up clinically at month 1, 3, 6 and 12 and annually. Stent patency was evaluated with carotid Doppler USG at month 1, 6, 12 and annually. CTA was performed for patients who were suspected to have restenosis. In-stent peak flow rate of ≥ 224 cm/s on Doppler USG and ≥ 50% stenosis on CTA were considered as restenosis.
DW-MRI
Cerebral DW-MRI images were obtained using a 1.5 Tesla Magnetom Sonata (Siemens, Erlangen, Germany). Cerebral MRI (DWI and ADC (apparent diffusion coefficient)) maps of patients were compared before and after CAS by an experienced interventional neurologist (E.S.G). New ipsilateral hyperintense DW-MRI lesions not seen before CAS were considered as silent cerebral embolism (Figure 1). The diffusion-weighted sequence was acquired with three different b values (b = 0.500, and 1000 s/mm2). A positive DWI scan was defined as high signal on the b1000 image. In cases with a lesion on DWI, we also reviewed the ADC map and noted whether high signal areas on the b1000 image showed a low, high or normal signal on the ADC map when comparing the affected area to the corresponding contralateral area. Furthermore, we assessed whether the lesions present on DWI were also present on the T2 image.
Statistical analysis
The data obtained in this study were recorded in SPSS 24.0 (Armonk, NY: IBM Corp.) software. The categorical variables were expressed as numbers and percentage while continuous variables were expressed as means and standard deviation. The Shapiro-Wilk test was used to analyze the concordance of the continuous variables with a normal distribution. For inter-group comparison, Student’s t-test was used for normally distributed parameters while the Mann-Whitney U test was used for other parameters that did not have a normal distribution. To analyze the categorical variables, the χ2 test or Fisher’s test was used. Optimal discriminant analysis was used to determine whether ‘age, predilatation balloon diameter, stent type, ipsilateral cranial microemboli’ CAS status. P < 0.05 was considered to be statistically significant.
Results
When the baseline characteristics of the classical and non-classical CAS groups were compared, the mean age of the patients in the classical CAS group was found to be statistically significantly higher (mean age in the non-classical and classical CAS groups, respectively, was 68.74 ±8.9 versus 71.34 ±10.2) (p = 0.012). No difference was found between the two groups as regards the other clinical characteristics (Table I).
Table I
Clinical characteristics of the study groups
| Variable | Non-classical CAS (n = 237) | Classical CAS (n = 130) | P-value |
|---|---|---|---|
| Age [years] mean ± SD | 68.74 ±8.9 | 71.34 ±10.2 | 0.012 |
| Male, n (%) | 188 (79.3) | 99 (76.2) | 0.605 |
| Hypertension, n (%) | 158 (66.7) | 91 (70.0) | 0.513 |
| Diabetes mellitus, n (%) | 87 (36.7) | 56 (43.1) | 0.232 |
| Coronary artery disease, n (%) | 171 (72.2) | 86 (66.2) | 0.230 |
| Peripheral artery disease, n (%) | 8 (3.4) | 10 (7.7) | 0.067 |
| Smoking, n (%) | 86 (36.3) | 43 (33.1) | 0.538 |
| Chronic renal failure, n (%) | 9 (3.8) | 3 (2.3) | 0.443 |
| Symptomatic ICA stenosis | 109 (46.0) | 72 (55.8) | 0.073 |
| LDL [mg/dl] | 111.6 ±43.0 | 109.8 ±37.7 | 0.843 |
| Statin intake | 217 (91.6) | 118 (90.8) | 0.797 |
| Drug resistance*: | |||
| Absent | 216 (91.1) | 117 (90.0) | |
| ASA | 2 (0.8) | 3 (2.3) | 0.169 |
| Clopidogrel | 19 (8.0) | 10 (7.7) | |
As for the procedural characteristics, open-cell stents were used more often in the non-classical stenting group while the closed and hybrid cell stents were used more often in the classical CAS group, which was statistically significant (p < 0.001). Diameter of balloon used for predilatation was already larger in the non-classical CAS group. Other procedural characteristics were similar between the two groups (Table II).
Table II
Procedural characteristics of the study groups
Discriminant analysis was performed for variables that were significant in terms of ipsilateral asymptomatic embolism between the two groups. According to the discriminant analysis, predilation balloon diameter was the best discriminator (Wilks’ lambda = 0.830, F = 72.629) followed by stent type (Wilks’ lambda = 0.935, F = 24.690) (Table III).
Table III
Discriminant function analysis using variables that had p < 0.05 in study groups
| Variable | Wilks’ lambda | F | df1 | df2 | Sig. |
|---|---|---|---|---|---|
| Age | 0.987 | 4.762 | 1 | 355 | 0.030 |
| Predilatation balloon diameter | 0.830 | 72.629 | 1 | 355 | 0.000 |
| Stent type | 0.935 | 24.690 | 1 | 355 | 0.000 |
| Cranial microemboli | 0.990 | 3.496 | 1 | 355 | 0.062 |
When the two groups were compared as for periprocedural asymptomatic ipsilateral cranial microembolism, which was the primary endpoint of our study, it was detected in 25 (10.5%) patients in the non-classical CAS group and 24 (18.5%) patients in the classical CAS group. On cranial DWI-MRI, periprocedural asymptomatic ipsilateral cranial embolism was detected statistically significantly less in the non-classical CAS group compared to the classical CAS group (p = 0.033) (Table IV).
Discussion
In this study, periprocedural ipsilateral cranial microembolism rates were compared with DW-MRI findings between the classical CAS procedure performed with suboptimal predilatation and postdilatation if needed and the non-classical CAS procedure performed with optimal predilatation but without postdilatation. The study findings demonstrated that the asymptomatic periprocedural cranial embolism rate was lower in the non-classical CAS group than in the classical group.
Despite large-scale randomized trials, safety of CAS is still controversial [1, 14]. Periprocedural stroke associated with CAS is still more prevalent than CEA [1, 14]. Stroke and transient ischemic attack after CAS are rare complications observed at high-volume and experienced centers [15]. Symptomatic or asymptomatic periprocedural cranial embolism is one of the most important limitations of CAS [16, 17]. Silent cranial embolism associated with CAS was demonstrated to cause dementia, cognitive decline [3] and even ischemic stroke in the subsequent years [4].
CAS-associated asymptomatic cranial embolism cannot be detected with neurological examination [18]. DW-MRI is a very sensitive method to detect cranial lesions that develop during CAS [5, 6]. The rate of silent cranial embolism associated with CAS and detected on DW-MRI was reported to be up to 40% in some series [19]. Thirty percent of these embolic events are observed in the contralateral hemisphere [20]. Unless an embolic protection method is used (unprotected), the rate of cranial embolism is 45%, which can be reduced to 33% with an embolic protection method [2, 9].
CAS has two primary goals: 1. to create sufficient lumen patency and 2. reduce the risk of potential embolism. The criteria to achieve carotid artery lumen patency are different from those of coronary arteries. If the carotid artery plaque is strained too much with a large balloon, carotid sinus reflex (bradycardia, asystole, hypotension) and plaque rupture/embolism may develop [21]. The techniques for CAS vary across centers; nevertheless, the main goal is to achieve sufficient lumen patency (< 30% residual stenosis) without causing hemodynamic and embolic complications. While achieving these goals, cranial embolism of patients is evaluated with clinical findings even in large-scale studies. Most of the periprocedural cranial embolisms, however, are asymptomatic. Therefore, we think that it is more reliable to test the technique with cranial DW-MRI, which is more sensitive to detect cranial embolism.
Even though there are several causes of cranial embolism associated with CAS, TCD studies have demonstrated that ipsilateral embolism mostly occurs during the procedural steps [10]. Particularly balloon postdilatation after stent deployment increases periprocedural cranial embolic events [22]. Hence, the less often we perform balloon dilatation, the fewer embolic complications will develop. Many centers perform the CAS procedure by first using a small balloon, then deploying a stent, followed by postdilatation, in which the balloon is inflated twice. This increases the risk of plaque rupture and plaque prolapse. In our non-classical method, however, a space is created for the stent to appose to the wall of the carotid artery well with optimal predilatation. Then the self-expandable stent settles on the artery wall with the closest diameter to its anatomy. Thus, there is no need for postdilatation, which is associated with a higher rate of plaque prolapse and embolic complications.
In our study, an open-cell stent design was used more often in the non-classical CAS group. As the self-expandable carotid stent design changes, its mechanical features also change [23]. As the space between stent struts increases, the risk of late neurologic events also rises. In a study conducted by Park et al. in 2013, the rate of new ischemic lesions detected by cranial DW-MRI after CAS performed with the open-cell stent design was found to be significantly higher than that in those procedures performed with the closed-cell stent design [24]. We think that the use of open-cell stents more often in the non-classical group of our study might have affected the increase in the rate of cranial embolism in this group. Therefore, we are of the opinion that this significant finding will not influence the conclusion of our study.
In our study groups, the predilatation balloon diameter was 3.0–4.0 (mean: 3.42 ±0.4) mm in the classical group. The pressure for balloon inflation was up to 8–10 atm, which were the maximum nominal values. Predilatation was performed in the non-classical group using balloons with a diameter of 4.0–5.0 (mean: 4.52 ±0.5) mm at nominal pressure values of 10–14 atm. Inflation of 4.0 mm balloons in the non-classical group at a pressure that was above the nominal values brings the balloon diameter to > 4.0 mm. Therefore, the balloon diameters were different in the two groups as different inflation pressures were applied to inflate 4.0 mm balloons. In the non-classical method, during the optimal predilatation procedure with a balloon, when the indentation on the balloon disappeared when the pressure inside the balloon was in the range of 10–14 atm or when 14 atm was reached, inflation was stopped. In this way, aggressive balloon dilatation was avoided.
In our study, the mean age of the patients in the classical CAS group was 2.6 years higher. The rate of all interventional procedures increases with age. We do not know to what extent the difference of 2.6 years of age between the groups might have affected the results of our study.
In CAS, patients, lesions and techniques vary in time as in all interventional procedures. The experience of centers and development of materials used are also in parallel with such change. We think that operators can follow up the CAS-associated complications more sensitively and change their techniques accordingly with the use of not only clinical examinations but also sensitive examinations such as DW-MRI. We also think that this can be possible if the same technique is used for similar lesions at experienced centers.
Our study had certain limitations. It was a retrospective and single-center study. Different stent cell designs with varying strut spaces from different brands were used in our study. Intravascular ultrasonography could have been performed to rule out the complications associated with the selected carotid balloon/stent diameter and balloon/stent deployment (plaque prolapse…).
Conclusions
The rate of periprocedural asymptomatic ipsilateral cranial embolism detected on cranial DW-MRI is lower in the CAS procedures performed with optimal predilatation but without postdilatation after stent deployment compared to the CAS procedures performed with suboptimal predilatation and postdilatation after stent deployment. There is a need for further prospective, multi-center studies on this matter with a higher number of patients.