Introduction
Anovulation accounts for about 20% of infertility [1]. Around 85% of anovulatory infertilities have normal gonadotropin levels and are classified as type 2 anovulation according to World Health Organization classification [2]. Most of the anovulation is due to polycystic ovary syndrome (PCOS) [3].
Guidelines recommend clomiphene citrate (CC) as first-line ovulation induction (OI) in women with type 2 anovulation or PCOS [2, 3].
Clomiphene citrate is an anti-oestrogen, non-steroidal compound, which blocks oestrogen receptors at the hypothalamus, releasing it from negative feedback, and augmenting GnRH release. Subsequently, the pituitary production of gonadotropins increased, resulting in follicular growth and ovulation [4].
The ovulation and pregnancy rates are 70–85% and 40–70%, respectively, when CC is used for OI [5]. About 15% of anovulatory women do not respond to CC (CC resistance) [3]. Failure to achieve pregnancy despite achieving ovulation after CC treatment is defined as CC failure [2, 4].
The anti-oestrogenic effect of CC on the endometrium is an important cause of CC failure [6]. Oestrogen is necessary for endometrial development during the follicular phase of the menstrual cycle [7].
The anti-oestrogenic effect of CC impairs endometrial development, and endometrial biopsies from women on CC show decreased number and diameter of endometrial glands, with delayed glandular maturation [8].
The poor endometrial development appears by ultrasound as thin endometrium. The oestrogen-mediated development of the endometrium is dependent on endometrial blood flow. Vasodilators were used previously as a potential solution to improve endometrial blood flow and thickness in infertile women with CC failure [9]. Sildenafil citrate (SC) inhibits phosphodiesterase-5 (PDE5) and cGMP-dependent protein kinases (PKGs), and maximizes the effect of nitric oxide (NO), thus facilitating smooth muscle cell relaxation [10]. Sildenafil citrate could improve uterine blood flow and induce endometrium proliferation [11]. Therefore, this study was designed to evaluate SC as an adjuvant to CC for OI in PCOS women.
Material and methods
A total of 595 infertile PCOS women were recruited for this crossover randomized controlled study, which was conducted from January 2018 to December 2019 after approval by the institutional ethical committee and after informed consents in accordance with the Helsinki declaration.
The study was completed with the final analysis of the data of 432 PCOS women, [163 were excluded because of non-matched inclusion criteria (141) and refusal to partici-pate (22)].
Inclusion criteria include infertile PCOS women (according to Rotterdam’s Criteria), aged 21–35 years, non-smokers, without other causes of infertility, and no contraindications for SC.
Women with congenital uterine anomalies, fibroid, adenomyosis, endometrial polyp(s), Asherman’s syndrome, chronic use of non-steroidal anti-inflammatory (NSAIDs), causes of infertility other than PCOS, and contraindications of sildenafil were excluded from this study.
The European Society for Human Reproduction/American Society of Reproductive Medicine (ESHRE/ASRM) criteria were used to diagnose PCOS [12]. The polycystic ovaries were diagnosed by the threshold for polycystic ovary morphology ≥ 20 follicles per ovary and/or an ovarian volume ≥ 10 mL on either ovary, ensuring that there are no corpora lutea, cysts, or dominant follicles [13].
According to the ESHRE/ASRM recommendation, causes of hyperandrogenism such as Cushing`s syndrome, late-onset congenital adrenal hyperplasia, and androgen-secreting tumours were excluded before diagnosing PCOS [14–16].
The contraindications of SC include sickle cell anaemia, uncontrolled either high or low blood pressure, cardiac women (i.e. hypertrophic cardiomyopathy, mitral or aortic stenosis, and chronic heart failure), and chronic liver disease.
Participants were randomly assigned to 2 groups (216 women in each group): clomiphene/sildenafil (C/S) group and CC group.
Participants in the C/S group received CC 100 mg/daily from the 2nd to 6th day of the menstrual cycle for OI plus oral SC 20 mg 12-hourly for 10 days starting from the 2nd day of cycle (as an adjuvant to CC).
Participants in the CC group received CC 100 mg/daily from the 2nd to 6th day of the cycle for OI.
Participants were subjected to transvaginal sonography (TVS) on the 9th and 13th day of the menstrual cycle, and when the dominant follicle reach 16 mm diameter the resistance index (RI), pulsatility index (PI), and maximum velocity (T-max) of sub-endometrial, uterine, and ova-- rian vessels were measured using TVS-Doppler.
Participants were examined using TVS on the 21st day of the cycle for detection of ovulation and after a positive pregnancy test for documentation of clinical pregnancy.
Participants with negative pregnancy tests were given 2 months rest without OI, followed by the crossover of OI medication between the 2 studied groups (145 women shifted to C/S protocol, and 126 women to CC protocol).
Crossover OI means participants in the C/S group without any documented ovulation after 3 cycles of OI received the CC protocol of OI, and participants in the CC group without any documented ovulation after 3 cycles of OI received the C/S protocol of OI. The crossover results were assessed by TVS, TVS-Doppler, and pregnancy test (as described previously).
The side effects related to SC or CC were assessed using an objective scaling scoring system: 0 (no side effects), 1 (mild side effects, without affecting lifestyle), 2 (severe side effects, but controlled by another method), 3 (severe, non-controlled side effects and participant still agreeing to continue medication), 4 (severe, non-controlled and participant not agreeing to continue medication). Collected data were analysed to evaluate SC as an adjuvant to CC for OI in PCOS women.
Sample size justification
The required sample size for this study was calculated using G Power software version 3.1.9.7 for sample size calculation, setting the α-error probability at 0.05, power (1-β error probability) at 0.95%, and effective sample size (w) at 0.5. An effective sample ≥ 210 PCOS-women in 2 studied groups was needed to produce a statistically acceptable figure.
Statistical analysis
Collected data statistically analysed using Statistical Package for Social Sciences (SPSS) version 20 (Chicago, IL, USA). Numerical variables were presented as mean and standard deviation (±SD), while categorical variables were presented as number (n) and percentage (%). The chi-square test (χ2) and Student’s t-test were used for the analysis of qualitative and quantitative variables, respectively. p < 0.05 was considered significant.
Results
During the first 3 months, the follicular maturation and ovulation were significantly higher among C/S (49.5% and 45.8%, respectively) compared to the CC group (34.3% and 29.2%, respectively) (p = 0.03 and 0.01, respectively). The endometrial thickness was significantly higher among C/S compared to the CC group (9.6 ±1.2 vs. 8.7 ±1.0 mm, respectively, p = 0.003). The chemical and clinical pregnancy rates were significantly higher among C/S compared to the CC group (39.8% and 36.6% vs. 25% and 18.98%, respectively) (p = 0.01 and 0.001, respectively) (Table 1).
Table 1
Follicular maturation, ovulation, endometrial thickness, and chemical and clinical pregnancies of the 2 studied groups in the first 3 months
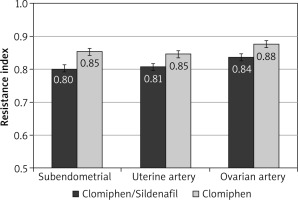
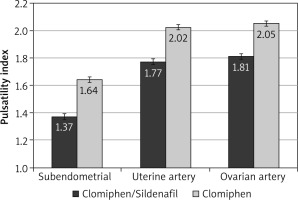
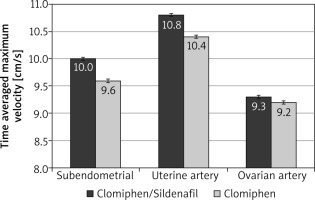
During the first 3 months, the mean RI was significantly lower among the C/S group compared to the CC group for sub-endometrial arteries (0.8 vs. 0.85, respectively), uterine artery (0.81 vs. 0.85, respectively), and ovarian artery (0.84 vs. 0.88, respectively, p < 0.001) (Fig. 1). The mean PI was also significantly lower among the C/S group compared to the CC group for sub-endometrial arteries (1.37 vs. 1.64, respectively), uterine artery (1.77 vs. 2.02, respectively), and ovarian artery (1.81 vs. 2.05, respectively, p < 0.001) (Fig. 2). The mean T-max was significantly higher among the C/S group compared to the CC group for sub-endometrial arteries (10 vs. 9.6 cm/s, respectively), uterine artery (10.8 vs. 10.4 cm/s, respectively), and ovarian artery (9.3 vs. 9.2 cm/s, respectively, p < 0.001) (Fig. 3).
After the crossover of OI medication, the follicular maturation and ovulation were significantly higher among the C/S group (43.4% and 41.4%, respectively) compared to the CC group (30.2% and 28.6%, respectively) (p = 0.02 and 0.02, respectively). The endometrial thickness was significantly higher among the C/S group compared to the CC group (9.1 ±1.3 vs. 8.2 ±1.0 mm, respectively, p = 0.007). The chemical and clinical pregnancy rates were significantly higher among the C/S group compared to the CC group (36.6% and 33.1% vs. 23.8% and 20.6%, respectively) (p = 0.02 and 0.01, respectively) (Table 2).
Table 2
Follicular maturation, ovulation, endometrial thickness, and chemical and clinical pregnancies of the 2 studied groups after crossover of ovulation induction medication
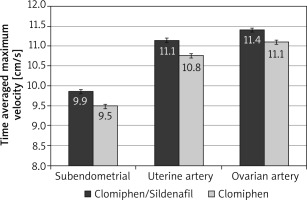
After the crossover of OI medications, the mean RI was significantly lower among the C/S group compared to the CC group for sub-endometrial arteries (0.81 vs. 0.85, respectively), uterine artery (0.82 vs. 0.86, respectively), and ovarian artery (0.85 vs. 0.89, respectively, p < 0.001) (Fig. 4). The mean PI was also significantly lower among the C/S group compared to the CC group for sub-endometrial arteries (1.53 vs. 1.73, respectively), uterine artery (1.66 vs. 1.88, respectively), and ovarian artery (1.69 vs. 1.92, respectively, p < 0.001) (Fig. 5). The mean T-max was significantly higher among the C/S group compared to the CC group for sub-endometrial arteries (9.9 vs. 9.5 cm/s, respectively), uterine artery (11.1 vs. 10.8 cm/s, respectively), and ovarian artery (11.4 vs. 11.1 cm/s, respectively, p < 0.001) (Fig. 6).
Fig. 4
The mean resistance index of the 2 studied groups after crossover of ovulation induction medications
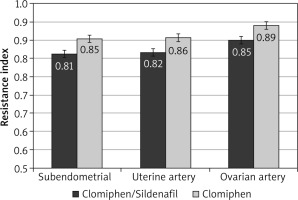
Fig. 5
The mean pulsatility index of the 2 studied groups after crossover of ovulation induction medications
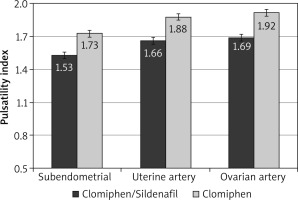
Fig. 6
The mean time-averaged maximum velocity of the 2 studied groups after crossover of ovulation induction medications
The side effects reported in this study were mild (score 1, not affecting lifestyle) and were significantly more frequent among the C/S group compared to the CC group. Headache was the commonest side effect (49.1% vs. 23.6%, respectively, p = 0.0001) followed by tachycardia (12.96% vs. 4.16%, respectively, p = 0.002), and hypotension (5.6% vs. 1.38%, respectively, p = 0.01) (Table 3).
Discussion
A total of 595 infertile PCOS women were recruited for this crossover randomized controlled study, and it was completed with the final analysis of the data of 432 PCOS women.
Participants were randomly assigned into 2 groups: the C/S group (who received CC for OI plus SC as an adjuvant to CC) and the CC group (who received CC for OI). The collected data were analysed to evaluate SC as an adjuvant to CC for OI in PCOS women.
The chemical and clinical pregnancy rates were significantly higher among C/S compared to the CC group (p = 0.01 and 0.001, respectively) during first the 3 months and after the crossover of OI medications (p = 0.02 and 0.01, respectively).
Fahmy et al. compared the use of SC as an adjuvant to CC during OI, and found the use of SC as an adjuvant to CC during OI improved the endometrial thickness, follicular number, and pregnancy rate [17].
Mohamed, compared the use of SC as an adjuvant to letrozole for OI in PCOS and found the endometrial thickness and clinical pregnancy rate were significantly higher among the SC/letrozole group compared to letrozole alone (12.7 mm and 55% vs. 9.8 mm and 15%, respectively) [18].
In addition, Mohamed, found the pregnancy risk ratio was 3.7 higher in the SC/letrozole group compared to the letrozole alone [18].
However, Check et al. found that neither vaginal oestrogen nor sildenafil significantly improved the endometrial thickness or endometrial blood flow in frozen embryo transfer cycles [19].
The endometrial thickness in this study was significantly higher among the C/S group compared to the CC group during the first 3 months (p = 0.003) and after the crossover of OI medication (p = 0.007).
Paulus et al. also reported that the use of SC during ART improves the uterine artery blood flow and endometrial thickness in women with thin endometria [20].
Moreover, Fisch et al. studied the effect of SC on endometrial thickness in 148 couples with unexplained infertility and found that SC significantly increases endometrial thickness when used from day 8 to day 13 of the cycle [21].
The effect of SC on endometrial thickness using different routes has been reported. Sher and Fisch, evaluated in a preliminary cross-over study the effect of Vaginal Sildenafil (VS) on the in vitro fertilization (IVF) outcome after multiple IVF failures attributed to poor endometrial thickness in women with normal ovarian reserve and at least 2 prior IVF cycles [22]. Sher and Fisch, found that VS improved the endometrial development in 70% of studied women [22].
Takasaki et al. examined whether thin endometrium can be improved by increasing uterine radial artery blood flow using different vasodilators, and they found significant improvement of endometrial thickness and uterine radial artery RI after the use of vasodilators in women with thin endometria [23].
Thus, the use of sildenafil cannot be expected to help all women with thin endometria. Women with intractable damage of basal endometria may be less likely to respond to increased uterine blood flow caused by sildenafil. In addition, the effect of sildenafil on the endometrium requires an adequate oestrogen level [24].
The mean RI and PI of sub-endometrial, uterine, and ovarian arteries was significantly lower among C/S compared to the CC group during the first 3 months, and after the crossover of OI medications in this study.
Das et al. and Malinova et al. investigated the role of combined VS and CC vs. CC alone on endometrial thickness, endometrial volume, and endometrial Doppler indices on the day of hCG, during intrauterine insemination (IUI), and found that the use of VS as an adjuvant to CC during OI achieved significantly higher endometrial thickness, with reduced uterine artery vascular resistance and increased pregnancy rate [25, 26].
Ashoush and Abdelshafy, studied SC as an adjuvant to CC for OI in PCOS women with CC failure and found that the use of SC as an adjuvant to CC for OI after CC failure was associated with significantly higher clinical pregnancy rates, endometrial thickness, and improved sub-endometrial blood flow indices [27].
Li et al. in a meta-analysis, studied the effect of SC on infertile women with thin endometria and found that the endometrial thickness and pregnancy rate were significantly higher in the SC group than controls [28]. In addition, they found that the radial artery RI was significantly lower in the SC group than controls [28].
Li et al. concluded that SC improves the endometrial thickness and pregnancy rate in infertile women with thin endometria [28].
A Cochrane review found the vasodilators had a beneficial effect on endometrial thickness and slightly improved the clinical pregnancy rate (RR 1.45; 95% CI: 1.19–1.77) [29].
The side effects reported in this study were mild (score 1) and were significantly more frequent among the C/S group compared to the CC group. Headache was the commonest side effect (p = 0.0001) followed by tachycardia (p = 0.002) and hypotension (p = 0.01).
Fahmy et al. reported headache as the most common side effect with SC (20%), followed by flushing (11.4%), blurring of vision (5.7%), and gastrointestinal disorders (5.7%) [17]. The side effects reported with SC are usually mild to moderate, dose-related, and include headache, flushing, blurring of vision, nausea, and dyspepsia [30–31].
This study found that SC as an adjuvant to CC for OI in PCOS women increases the chemical and clinical pregnancy rates. It also improves the endometrial thickness and ovulation rate through improved endometrial and ovarian Doppler indices.
Women who refused to participate and/or give consent was the only limitation faced during this study. Further, larger studies are needed to confirm the beneficial effect of sildenafil when used by different routes (oral or vaginal) on the endometrial pattern, ovulation, and pregnancy rates.