Coronary artery disease (CAD), due to common risk factors, often accompanies aortic stenosis (AS) [1]. CAD occurs in 50–75% of patients undergoing transcatheter aortic valve implantation (TAVI) [1]. However, there are no data on the necessity and the extent of revascularization in CAD patients referred for TAVI. According to the ESC/EACTS guidelines for myocardial revascularization published in 2018, percutaneous coronary intervention (PCI) should be considered in patients with stenoses > 70% in proximal segments of coronary arteries, undergoing TAVI [2]. Optimal timing (before, simultaneously or after TAVI) and the mode of revascularization have not yet been established [2].
The 83-year-old patient with exertional dyspnea (NYHA III) and stenocardia (CCS III) was admitted to our center due to severe dyspnea.
Echocardiography revealed severe aortic stenosis (mean pressure gradient 40 mm Hg, aortic valve area 0.7 cm2) and preserved left ventricular systolic function (LVEF 50%). Coronary angiography showed 60% aorto-ostial stenosis in the left main artery (LM) (Figure 1 A) as well as 80% aorto-ostial lesion in the right coronary artery (RCA) (Figure 1 B).
Figure 1
A – The left coronary artery (LCA) angiogram shows ostial stenosis, B – the right coronary artery (RCA) angiogram reveals an ostial lesion, C – LCA angiogram after stent placement and postdilatation, D – RCA angiogram after stent implantation, E – Aortography during valve positioning, F – Aortography after valve implantation shows no paravalvular leak and patent coronary arteries
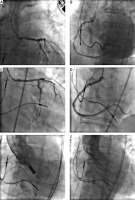
Due to the high risk of surgical aortic valve replacement (AVR) + coronary artery bypass grafting (EuroSCORE II 6.93%, STS 8.06%), the patient was referred by the Heart Team for percutaneous revascularization and TAVI. Because of confirmed allergic reactions to iodinated contrast media as well as chronic kidney disease (stage G3a) it was decided to perform transfemoral TAVI and PCI of two ostial lesions in the LM and RCA in the same setting.
The procedures were performed under local anesthesia after corticosteroid and antihistamine premedication. PCI was carried out via the left femoral access. The RCA ostial lesion was predilated (2.5 × 10 mm scoring balloon and a 3.5 × 15 mm non-compliant balloon). Subsequently, a sirolimus-eluting 4.0 × 15 mm stent (Alex Plus, Balton, Poland) was implanted and post-dilated with a 4.0 × 12 mm non-compliant balloon. In the LM stenosis, a sirolimus-eluting 4.0 × 15 mm stent (Alex Plus, Balton, Poland) was implanted afterward, using the direct stenting technique. The proximal edge of the stent was post-dilatated with a 4.0 × 15 mm balloon (Figures 1 C, D).
Access to the right femoral artery was achieved via surgical cutdown. The predilation was done during rapid pacing (180/min) with the Valver Balloon (20 × 40 mm, Balton, Poland). The ACURATE neo M valve system (Boston Scientific, USA) was introduced and implanted in a typical way (Figure 1 E). Control aortography revealed intact flow in the coronary arteries (Figure 1 F). There was no leak in the immediate echocardiography and the maximal transaortic gradient was 7 mm Hg. The whole procedure time was 1 h 50 m, the entrance surface dose was 1360 mGy and the total contrast volume was 170 ml. Acute contrast injury and allergic reaction were not observed. The patient was discharged on day 3 after the procedure.
Three months after the procedure, the patient felt well. She did not report dyspnea or stenocardia.
Although CAD is present in up to three-quarters of patients with AS, currently, there are no firm data regarding optimal revascularization strategies and the benefits of PCI before TAVI [1, 2]. However, significant narrowings of the coronary arteries, especially aorto-ostial lesions, increase the risk of peri-operative ischemia during rapid pacing or in case of sudden hemodynamic deterioration in the perioperative period [3, 4].
In patients undergoing TAVI and requiring coronary revascularization, optimal timing of PCI is also disputed. Two meta-analyses of observational studies did not show any advantage of prior revascularization compared to revascularization during the same procedure in terms of mortality and incidence of acute kidney injury [3, 4]. Potential benefits resulting from the same setting strategy are associated with reducing the hospitalization time, shortening the period in which the underlying disease, i.e. AS, is untreated, and reducing the risk of complications associated with vascular access (using the same vascular access) [3, 4].
The described case reveals that it is possible to perform TAVI and multivessel PCI in selected patients. The potential benefits of simultaneous PCI and TAVI, especially reduction in total hospitalization time, are particularly important for elderly patients.








