The incidence of neuroendocrine tumors (NET) is 2.5–5 per 100 000 population. Up to 20% of patients with NET develop carcinoid syndrome (CS), and in 40–50% of those carcinoid heart disease (CHD) is reported (0.27 per 100 000 population per year) [1]. Development of CS and CHD is determined by numerous vasoactive substances (serotonin, kallikrein, bradykinin, histamine, prostaglandins, etc.) which are produced by NET [2]. Primary ovarian carcinoid is a rare disease and accounts for 0.3–1.0% of all carcinoid tumors and up to 0.1% of ovarian tumors [3]. Venous drainage from ovaries, as from other paired organs, bypasses the liver and enters the vena cava inferior, which is the main reason for early development of CS and manifestation in ovarian carcinoids. Typical clinical symptoms of CS include flushing (90%), diarrhea (70%), wheezing (15%), fatigue, hypotension and arrhythmias [4]. Treatment of CS is a challenge due to often late diagnostics, life-threatening carcinoid crises and lack of established guidelines for surgical treatment of ovarian tumors, associated with CHD [5].
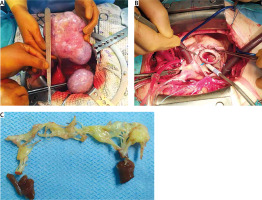
A 64-year-old female patient was referred to our unit in September 2019 complaining of dyspnea on minor physical exertion, limb swelling, flushes and diarrhea 6 months prior to admission. Holter monitoring revealed sinus rhythm with frequent ventricular arrhythmia (7754 ventricular extrasystoles). Transthoracic echocardiography (TTE) showed mild regurgitation of the aortic valve, mitral valve and pulmonary artery valve (PV), severe tricuspid valve (TV) regurgitation, right ventricle enlargement, left ventricular ejection fraction of 68%, tricuspid annular plane systolic excursion (TAPSE) of 20 mm, right ventricular ejection fraction of 41%, systolic pulmonary artery pressure of 33 mm Hg, with enlargement of the pulmonary artery. Cardiac magnetic resonance imaging confirmed right ventricle enlargement (4 × 3.8 cm/m2, end-diastolic volume – 257 ml, end-systolic volume –150 ml), right ventricular ejection fraction was 41%. Ovarian tumor was revealed 2 months prior to admission to our hospital when computed tomography showed uterus enlargement (57 × 68 × 63 mm) and two cystic-solid tumors 140 × 150 × 85 mm and 95 × 60 × 75 mm in place of right and left ovaries, respectively. Laboratory findings included elevation of tumor markers CA-125 – 136.3 (N: 0–30) (U/ml), CEA – 4.24 (N: 0–3.8) (U/ml), CA-15-3 – 27.1 (N: 0–22) (U/ml), as well as the nonspecific NET marker chromogranin A – 706.9 ng/ml (N: 0–100). CS was diagnosed and after multidisciplinary discussion with the oncology team it was decided to perform simultaneous TV replacement and panhysterectomy. The patient received intravenous octreotide in the dose of 50 µg/h starting from 6 hours preoperatively. Abdominal surgery (panhysterectomy) was performed through lower midline incision. Ovaries (left: 60 × 70 mm, right: 120 × 150 mm) had solid and tuberous structure. Upon completion of abdominal intervention cardiac surgery was performed via median sternotomy. At revision, fibrosis, retraction and reduced mobility of TV leaflets were found. TV was replaced with an Edwards Perimount Magna 27 mm bioprosthesis (Figure 1). Cardiopulmonary bypass time was 82 minutes, myocardial ischemia time was 50 minutes. Octreotide infusion was continued intraoperatively in the same dose, which provided stable hemodynamic parameters within normal values. The patient was extubated after 11 hours but remained in the intensive care unit for 6 days due to moderate respiratory failure and unstable hemodynamics that required continuous infusion of norepinephrine and milrinone. Intravenous octreotide was continued in the dose of 25 µg/h during the first three postoperative days (POD). After the surgery the patient developed atrial fibrillation paroxysm and cardiac tamponade, which were successfully treated. On POD 10 she also developed neurological deficit and was diagnosed with ischemic stroke. The stroke was managed conservatively and at the time of the discharge neurological symptoms has resolved. Embolic material was thought to originate from carotid arteries that had a degree of atherosclerosis with stenoses up to 30% on carotid ultrasound. TTE on POD 7: aortic valve, mitral valve and PV and tricuspid bioprosthesis – mild regurgitation without stenosis, systolic pulmonary artery pressure – 36 mmHg, LVEF – 61%, RVEF – 44%. Chromogranin A on POD 11 declined to 132.0 ng/ml. Positron emission tomography with 18F on POD 26 revealed no metabolic-active tumors or metastases. The patient was discharged on POD 36 in a satisfactory general state. Histopathological examination confirmed ovarian carcinoid, insular type (Figure 2). Immunohistochemical results were as follows: CK (AE1/AE3), synaptophysin and chromogranin A were positive; calretinin and α-inhibin were negative. The positive index of Ki67 was < 3%. The patient received no chemotherapy. Single photon emission computed tomography (SPECT/CT) with 99mTc-Tektrotyd was performed 1 year after surgery: no metastatic lesions were detected. During follow-up at 18 months after surgery, abdominal and pelvic magnetic resonance imaging with late gadolinium enhancement were performed: no abnormalities were detected. TTE revealed mild aortic valve, mitral valve and PV regurgitation, tricuspid bioprosthesis – trace regurgitation with effective orifice area 3 cm2, mean gradient 5 mm Hg. Chromogranin A further declined to 42.7 ng/ml. 30 months after surgery the patient was in good health and had class II heart failure according to the NYHA classification. The patient was physically and socially active with a good quality of life.
Figure 2
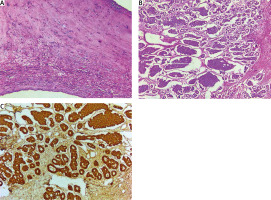
Histological examination. A – TV leaflet – focal fibrosis, hyalinosis, reactive lymphoid infiltration (hematoxylin and eosin staining, 50×); B – ovarian tumor – insular pattern (hematoxylin and eosin staining, 100×); C – ovarian tumor – diffuse intense immunoreactivity of chromogranin A (immunohistochemical staining, 100×)
Vasoactive substances that are released into the systemic circulation by NET lead to specific changes predominantly in the right heart – fibrosis and sclerosis of valve leaflets, chords, papillary muscles as a result of myofibroblasts, smooth muscle cells and extracellular components deposition – which in turn cause severe congestive heart failure [2]. Polychemotherapy and surgery carry significant cardiovascular risks, as well as a decline in quality of life. The most common treatment approach in gastrointestinal carcinoid complicated with CS and subsequent TV and PV degeneration is valve replacement with biological or mechanical prostheses. Radical primary tumor removal is often unfeasible due to late diagnosis and distant metastases. Patients are treated with palliative surgery, life-long symptomatic treatment and prolonged somatostatin infusions, aimed at carcinoid crisis prevention. Ovarian carcinoid is characterized by early onset of CS, which facilitates diagnostics and increases the probability of radical treatment. However, have been published only isolated cases of the staged approach to surgical treatment of CHD and primary ovarian tumor resection. It should be noted that the staged approach has major drawbacks in either cardiac or abdominal surgery performed first. A staged operation with cardiac surgery first (heart valve replacement) can be associated with carcinoid crisis events and bioprosthesis degeneration due to tumor persistence. Delay in primary tumor resection also carries the risk of disease progression. A staged operation with abdominal surgery first carries the risk of hemodynamic instability due to persistent heart disease. There are numerous publications describing successful simultaneous surgical treatment in patients with common cancers and concomitant heart diseases [6, 7]. It is remarkable that a simultaneous approach in case of NET and CHD does not carry the above-mentioned drawbacks of staged treatment. Moreover, in our case combination of panhysterectomy and TV replacement was associated with acceptable complexity and duration of surgery, mechanical lung ventilation time and blood loss. Taking this into consideration, simultaneous cardiac and oncological surgical treatment can be considered as a beneficial option for these patients. We would like to emphasize again the necessity of a multidisciplinary approach in treatment of NET with concomitant CS and CHD. Octreotide is a synthetic octapeptide that mimics natural somatostatin in its pharmacological properties. The role of octreotide in NET treatment is due to its direct and indirect antiproliferative effects. Direct effects involve binding to somatostatin receptors on the surface of tumor cells, thereby inhibiting secretion of vasoactive substances and hormones, inhibiting the cell cycle and inducing apoptosis. Indirect systemic effects include inhibition of tumor growth factor release, suppression of angiogenesis and immune system modulation. In this case we used continuous infusion of octreotide to decrease the release of tumor vasoactive substances as a carcinoid crisis prophylactic measure. It was shown that Ki67 proliferative index correlates with differentiation grade and survival in NET. According to the 2019 WHO classification Ki67 proliferative index < 3% corresponds to a G1 grade of well-differentiated gastroenteropancreatic NET with mitotic count < 2 per 2 mm2 and is associated with better prognosis. Previous studies showed that ovarian carcinoid cases with Ki67 ≥ 7.5% had significantly shorter survival as comparted to those with Ki67 < 7.5%. In our case Ki67 < 3% was associated with long-term survival after radical surgical treatment.
To our knowledge this is the first description of simultaneous TV replacement and panhysterectomy in a patient with primary ovarian carcinoid and CHD, associated with TV disease. We believe that simultaneous surgery represents an effective and contemporary approach in treatment of NET and concomitant CHD, and reduces the risks of cardiovascular complications, carcinoid crisis and tumor progression. Continuous infusion of octreotide pre-, intra- and postoperatively reduces the risks of surgical intervention. Other treatment options include somatostatin analogues and adjuvant polychemotherapy, depending on the presence of distant metastases.