Introduction
Despite on-going improvements in interventional and pharmacological therapy, ischemic heart disease remains a leading cause of death in modern societies, with chronic ischaemic heart failure (CIHF) as an important cause of reduced quality of life and disability [1–4].
The pathophysiology of acute and chronic heart failure is similar and is primarily associated with irreversible loss of viable myocardium, leading to impairment of contractile function. Although wide access to primary percutaneous coronary intervention (PCI) decreased the rate of “direct” myocardial infarction-related death, the number of CIHF patients has been increasing worldwide and their prognosis remains poor [2, 3, 5].
Optimal pharmacotherapy may prolong life and improve its quality [6, 7].
However, overall benefit arising from conservative treatments is likely to be “biologically” limited because, as of today, there is no clinically available feasibility for any meaningful myocardial tissue regeneration. One of the directions towards restoration of functionally effective heart muscle is through progenitor cell-mediated repair and regeneration. Progenitor cells have a self-renewal capability and they were found to be able to differentiate into specialized cells, including (at least in some conditions) cardiac myocytes and endothelial cells. A key concept in the regeneration hypothesis is to effectively deliver progenitor cells to the area of infarction and to keep them alive so that they can promote endogenous repair and regeneration by a paracrine effect and/or to direct them to transform into fully functional myocardial tissue. Thus, cell uptake and retention are fundamental for any effect of cell therapy.
There is evidence that progenitor cells may stimulate cardiac repair and regeneration by producing wide range of cytoprotective, anti-inflammatory and angiogenesis promoting factors leading to oxidative stress reduction, ventricle remodeling inhibition and recruitment of endogenous progenitor cells [8–10].
There are several types of cells that have been investigated in this field, including skeletal myoblasts, embryonic stem cells, cardiosphere-derived autologous stem cells, endothelial progenitor cells, bone marrow-derived mesenchymal stem cells (BMSCs), stromal vascular fraction containing primitive stem cells and pluripotent stem cells [11–14]. Although there is evidence that all of these cell lines may have regenerative potential, the type of cell, method of delivery and time from ischemic damage to cell transplantation have not been established clearly, though they seem to be key factors influencing the final pro-regenerative effect. To better understand the fate of progenitor cells after implantation, two main focus points of cell-derived regeneration therapy have been evaluated: a) cells’ biodistribution, including local retention, i.e. the amount of cells that are captured by myocardial tissue after transplantation (the higher the retention, the greater the chance for the activation regeneration process; and b) quantitatively measured long-term effects of cell-derived regeneration (i.e. left ventricle ejection fraction and volumes). Those parameters may be evaluated with different imaging methods including magnetic resonance imaging (MRI), positron emission tomography (PET) and single-photon emission computed tomography (SPECT) [15]. Each of these modalities has its strengths and limitations. This review focuses on SPECT – a technique that, amongst other techniques, may offer an optimal balance between advantages and limitations in the context of cell uptake imaging, evaluation of myocardial perfusion, and study of evolution of global and regional function.
SPECT, applied in everyday clinical practice, is widely available, easy to perform and provides feasibility to estimate precisely and repetitively left ventricle parameters [16]. Moreover, with the use of different tracers, SPECT enables one to track progenitor cells in-vivo in the early phase after transplantation [17].
It has been shown that as few as 2900 cells can be detected by SPECT without significant viability loss due to radiation [18]. SPECT may provide detailed information concerning localization and homing (Figure 1) and – using tracers with a long half-life – also migration of the transplanted cells [17]. Specifically , if the tracer used has a sufficiently long half-time (i.e. 111In with half-life of 2.8 days), it is also possible to track cells at several time points [19–21]. However, a long half-life of the tracer is inextricably linked to an important drawback of SPECT – radiotoxicity [22–24]. Another important limiting factor is time-dependent efflux (leak) of the label from the cells (Table I). For these reasons, SPECT may not be suitable for long-term tracking of progenitor cells (i.e. half-lives 99mTc, t1/2 = 6 h; 111In, t1/2 = 2.8 d). As compared to computed tomography or magnetic resonance imaging, clinical SPECT has somewhat lower spatial resolution (~7–15 mm) that can be an issue in precise signal localization [15].
Table I
Advantages and limitations of direct cell labelling
Figure 1
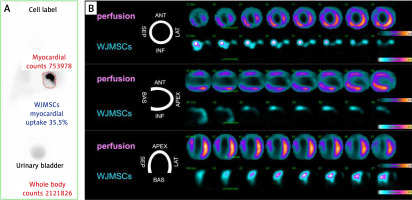
Essential role of single-photon emission computed tomography (SPECT) in evaluating the magnitude of cardiac uptake of multipotent stem cells and in determining the zone(s) of early cell homing in relation to the infarct injury: use of standardized Wharton jelly mesenchymal stem cells (WJMSCs, umbilical cord stem cells) as an advanced technology medical product (WJMSCs-ATMP). A 62-year-old man was admitted due to anterior ST-segment elevation acute myocardial infarction. Left anterior descending coronary artery proximal occlusion was treated successfully with thrombus aspiration (to minimize distal embolization and myocardial microcirculatory obstruction in the infarct zone) [104–106] and primary angioplasty with stent implantation. Six days later, consistent with the CIRCULATE-AMI Pilot Study Protocol, 30 × 106 standardized Wharton jelly pluripotent stem cells (50% labeled with 99mTc-sestamibi) were administered via the infarct-related-artery using a dedicated system for transcoronary delivery of cells and cell-based products (CIRCULATE Catheter, Protected Design No 72837, Patent Office of the Republic of Poland) [107]. Whole-body scintigraphy (A) performed 60 min after transcoronary WJMSCs-ATMP transplantation revealed a large-magnitude (35.5%) myocardial uptake of the WJMSCs-ATMP (red-line delineation; note that this WJMSCs-ATMP uptake exceeds, by ~7-fold, the uptake of prior-tested cell types such as CD34+ cells [32, 57, 72]. B shows the areas of WJMSCs-ATMP early homing (bottom rows labeled ‘WJMSCs-ATMP’ in the top, middle and bottom panel) in relation to regional myocardial perfusion by SPECT (top rows [“perfusion”] in the top, middle and bottom panel). Note WJMSCs-ATMP homing to the areas of severe perfusion defect in the anterior wall, septum and apex of the LV myocardium (myocardial infarct zones), consistent with a role of biologic mechanisms attracting WJMSCs to ischaemia-injured myocardium but not to normal myocardium [108]
Cell labeling protocols
The principles of labelling are similar for all nuclides. After harvesting cells are incubated with so-called ‘linker’ allowing the tracer to penetrate the cell membrane. For cell tracking in the heart, [In-111]oxine [19, 23, 25, 26], [In-111]tropolone [27, 28], and [Tc-99m] hexamethylpropyleneamine oxime [29–32] have been adopted.
99m-Technetium radioactive isotope bound to hexamethylpropyleneamine oxime (99mTc-HMPAO, 99mTc-extametazime, CERETEC) is the most widely used compound for viable cell labelling. This complex is lipophilic and can easily cross the cell membrane. When inside, in an alkaline environment, it changes into the hydrophilic ionic form and remains sequestered inside the cell. The complex efflux is constant, but this phenomenon prolongs the time of possible gamma camera acquisition. Nevertheless, cells labelled with 99mTc-HMPAO can be observed only in the first 24 h after administration, due to the relatively short half-time of 99mTc of 6 h. 99mTc-HMPAO that is released by cells is taken up by the liver and excreted via the intestines. Labelling cells with 99mTc requires expertise in handling blood-derived products, and does not significantly affect cell viability and functionality while reaching a high level of binding capacity (~40–69%) [33, 34]. For in-vivo tracking, the cultured cells are first trypsinized and incubated with Tc-99 with HMPAO linker for a 10–30 min period, then the cells are washed to eliminate any unbound radioactivity. Finally they are injected into the host. The time of incubation is a compromise between the need for a high rate of labelling efficiency and cell viability, and it usually does not exceed 30 min with cell viability deterioration < 2% [34–36].
The 111In-oxine is a complex of indium and three molecules of 8-hydroxyquinoline (oxine). The complex is lipid-soluble, and, similarly to 99mTc-HMPAO, and penetrates the cell external membrane by passive diffusion. After binding cytoplasmatic proteins, particles of 8-hydroxyquinoline are liberated and released out. Due to the long half-life of 111In (2.8 days) it is possible to track the transplanted cell up to 2 weeks. On the other hand, labelling cells with long half-life 111In-oxine may significantly impair the viability, proliferation and differentiation [23, 37]. Moreover, in-vivo cell tracking with 99mTc and 111In labelling is applicable for short-term analysis as it is difficult to detect whether the radiation intensity decrease is caused by radionuclide efflux from viable cells or it is associated with cell death or cell transfer to a remote location.
Cell tracking
Progenitor cell SPECT tracking feasibility has been tested in numerous animal models of myocardial infarction [19, 21, 25, 28, 38–52] that are summarized in Table II.
Table II
SPECT evaluation of myocardial uptake for cell therapies in preclinical studies of myocardial infarction
[i] 111In – indium-111, 99mTc – technetium 99m, HMPAO – hexamethylpropyleneamine oxime, 123I – iodine-123, MSCs – mesenchymal stem cells, IPSc – induced pluripotent stem cells, EPCs – endothelial stem cells, ESCs – embryonic stem cells, BM-MSCs – bone marrow mesenchymal stem cells, ADSCs – adipose tissue stem cells, HPCs – hematopoietic cells, iv – intravenous, im – intramyocardial, ic – intracoronary, ant.m. – anterior myocardium, inf.m. – inferior myocardium.
Brenner et al. demonstrated the feasibility of in vivo method for monitoring myocardial homing of transplanted cells in a rat myocardial infarction model using 111In-oxine-labelled CD34(+) hematopoietic cells. They found that viability of radiolabelled hematopoietic cells (HPCs) was impaired by 30% after 96 h, whereas proliferation and differentiation of cells were nullified after 7 days. The overall radioactivity detected in the heart was only about 1% [25].
Chin et al. examined the feasibility of 111In-oxine labelling of mesenchymal stem cells (MSCs) and single photon emission computed tomography imaging after intravenous administration in a porcine model of myocardial infarction. High initial MSC localization occurred in the lungs and no appreciable accumulation occurred in the myocardium. The authors conclude that 111In-oxine radiolabelling of MSCs is feasible, and in vivo imaging with SPECT provides a non-invasive method for sequentially monitoring cell trafficking with good spatial resolution [47].
Aicher et al. observed that after administration of 111In-oxine-labeled endothelial progenitor cells, the heart-to-muscle radioactivity ratio increased significantly in a myocardial infarction rat model, indicating increased homing of transplanted EPCs [48].
Garikipati et al., using pinhole gated SPECT-CT in a rat model, observed focal uptake of 99mTc-labeled fC-MSCs in the region of myocardial infarction. The uptake was associated with significant improvement in left ventricular ejection fraction 4 week after cell transplantation [31].
Tran et al. tracked 111In-oxine-labeled autologous BMSCs injected directly at the 1-month-old infarction site. One week later the myocardial retention of BMSCs was definitely higher in myocardial infarction than in the normal myocardial area (retention at 2 h: 63% vs. 25%, p < 0.001) and the estimated cardiac retention values were unchanged in both groups during the 7 days of follow-up [19].
Wisenberg et al. recorded an effective biological clearance half-life from the injection site of ~5 days for 111In-tropolone labelled bone marrow monocytes and stromal cells in a canine model [53].
Templin et al. applied dual isotope SPECT-CT imaging: 123I to follow donor cell survival and distribution, and 99mTC-tetrofosmin for perfusion imaging in a pig model of myocardial infarction. Additionally, sodium iodide symporter (NIS) transgene imaging was evaluated as an approach to follow in vivo survival, engraftment, and distribution of human-induced pluripotent stem cells. In vivo, viable NIS(pos)-hi pluripotent stem cells (PSCs) could be visualized for up to 15 weeks. Immunohistochemistry demonstrated that hiPSC-derived endothelial cells contributed to vascularization. Up to 12 to 15 weeks after transplantation, no teratomas were detected [50].
Dual isotope SPECT 111In-labeled/99mTc-sestamibi enables the imaging of both cells and perfusion deficit in the infarcted region simultaneously. Zhou et al. found that the 111In signal from the labelled stem cells overlaps the perfusion deficits identified from the 99mTc-sestamibi images. The 111In signal associated with the radiolabelled stem cells could be detected with SPECT of the heart for 96 h after engraftment [51].
Shen et al. used dual-tracer small-animal SPECT images to detect successfully 111In-labeled stem cells in the region of perfusion deficit assessed with 99mTc-sestamibi tracer. SPECT regional perfusion deficit coincided with the akinetic region on the MR image [52].
In several studies, combined, hybrid tomography has been applied for co-registration of structural and functional information within a single study. It has been demonstrated that the hybrid SPECT/CT system allows the combination of the exquisite anatomic details provided by CT with the functional, physiologic or metabolic information provided by molecular imaging.
The hybrid SPECT/CT system used by Sabondjian et al. was able to detect a signal of endothelial progenitor cells labelled with ¹¹¹In-tropolone within the zone of reduced perfusion delineated on first-pass perfusion CT in a canine model [27].
Kraitchman et al. detected focal and diffuse uptake of 111In-oxine-labeled mesenchymal stem cells in the infarcted myocardium in SPECT/CT images in the first 24 h after injection. The activity persisted until 7 days after injection. In contrast, MRI was unable to demonstrate targeted cardiac localization of MSCs, in part because of the lower sensitivity of MRI (~103 cells with SPECT versus ~105 cells with MRI) [21, 53, 54].
With the use of novel, nanoparticle(NP)-based labels (including iron oxide NPs, gadolinium-based NPs, manganese-based NPs, 19F-based NPs and SPIONs), MRI aims to reach high sensitivity, high spatial resolution and penetration depth for in vivo cell tracking [55].
Blackwood et al. developed a quantitative method to assess transplanted cell survival in myocardium using SPECT and 111In. The authors found that the measured half-time for transplanted cells was 74.3 h, and, when appropriate corrections (related to radiolabel leakage and extracellular 111In (e.g., after cell death)) were applied, the time was 71.2 h [28, 56].
Our group introduced SPECT-cMRI hybrid imaging, involving SPECT (a highly sensitive cell label) and magnetic resonance imaging (infarct) to evaluate the relationship between infarct size and progenitor cell uptake, including determination of the early homing zones [57].
How to enhance therapeutic cell retention
The low retention rate in most animal studies gave rise to a question of the mechanisms of cell washout and potential solutions leading to engraftment improvement.
Danoviz et al. injected directly in the infarct zone 99mTc-labeled adipose tissue-derived stem cells 24 h after MI using fibrin or collagen as the vehicle. The collagen group showed the highest radioactivity retention (26.8%) as compared to the fibrin group (13.7%) and control group (4.84%). The authors suggest that the low retention rate may be a result of washing out of cells from the myocardium through the lymphatic vessels and veins from the left ventricle into the lungs. Another finding was that intramyocardial injection of ASCs mitigates the negative cardiac remodeling and preserves post-MI ventricular function in rats, and these beneficial effects can be further enhanced by administering co-injection of ASCs with biopolymers [38].
Mitchell et al. reported that transplantation of 111In-tropolone-labeled endothelial progenitor cells into sustained occlusion infarcts resulted in a slower cell clearance half-life of 77.1 h (n = 18) versus reperfused – 59.4 h (n = 21). Sustained occlusion infarcts had longer cell retention in comparison to reperfusion whereas the timing of injection did not affect clearance rates [43].
Maureira et al. using dual 111In/99mTc-Sestamibi imaging observed cell engraftment in the MI area 48 h after stem cell transplantation. Interestingly, the authors also found that perfusion enhancement was sustained during the 6-month follow-up in the non-engrafted MI-areas from treated rats, whereas the engrafted ones, as well as the MI areas from control rats, exhibited progressive deterioration over time, suggesting a distant paracrine effect of transplanted cells [40].
Routes of cell administration to myocardium
During the first animal studies on cell retention, along with different cell types and methods of labelling, the way of transplantation was also examined. It has been found in numerous studies that cell delivery route can significantly influence the level of retention.
Forest et al. found that a significant 99mTc-labeled bone marrow stem cell fraction remained within the heart after intracoronary injection (6 ±1.7% of injected radioactivity at 24 h). With peripheral intravenous cell injection, no cardiac homing was observed at 24 h and cells were mainly detected within the lungs [42].
Although feasible, epicardial cell implantation appeared to be a complex and time-consuming procedure, requiring surgical technique. To simplify direct cell injection, Mitchell et al. proposed an endocardial approach. The authors found no significant difference between the endocardial (retention: 54%) and epicardial (retention: 57%) injection methods or the clearance kinetics, indicating that the injection strategies are comparable [39].
Barbash et al. examined different ways of cell delivery. They found that delivery by left ventricular cavity infusion results in drastically lower lung uptake, better uptake in the heart, and specifically higher uptake in infarcted compared with sham-MI hearts [44].
Hou et al. found significantly higher retention of 111In-oxine-labeled human peripheral blood mononuclear cells injected directly into myocardium (11%) as compared to intracoronary (2.6%) and retrograde coronary venous (3.2%) delivery [58].
Tossios et al. were another group investigating role of cell delivery method. They found that retention of 111In-labeled bone marrow cells after intracoronary infusion with or without balloon occlusion does not differ significantly (4.1% vs. 6.1% respectively) as opposed to direct intramyocardial injection (20.7%). Interestingly, dynamic SPECT during intracoronary injections showed rapid (20%) cell loss during balloon inflation and rapid (37%) cell loss after balloon deflation. After intramyocardial injection only slow linear cell loss was observed (9.7% per h) [45].
Kupatt et al. found, in a pig model of ischemia, that 1 h after reperfusion, 99Tc-HMPAO-labelled endothelial progenitor cells (eEPCs) engrafted to a 6-fold higher extent in the ischemic myocardium after retroinfusion than after intravenous application. Moreover, compared with medium-treated animals, retroinfusion of eEPCs decreased infarct size (35% vs. 52%) and improved regional myocardial reserve of the apical LAD region (SES 31% vs. 7%), whereas intravenous application displayed a less pronounced effect (infarct size 44%; SES 12%). Retroinfusion of an equal amount of neonatal coronary endothelial cells (rat) did not affect infarct size or regional myocardial reserve. Interestingly, the eEPC-dependent effect was detected at 24 h of reperfusion, suggesting an important role for enzyme-mediated cardioprotection [30].
It was hypothesized that a low engraftment rate may be influenced by a hostile environment for transplanted cells (hypoxia, inflammation etc.). Chan et al. investigated the theory of protective features of hydrogels. They found that hyaluronic acid-serum hydrogels markedly increase acute intramyocardial retention (∼6 fold), and promote in vivo viability, proliferation, engraftment of encapsulated stem cells and angiogenesis. The authors conclude that hyaluronic acid-serum hydrogels serve as ‘synthetic stem cell niches’ that rapidly restore the metabolism of encapsulated stem cells and promote stem cell engraftment and angiogenesis [59].
As shown in Table II, direct, intramyocardial cell injection provides the highest rate of in-tissue retention immediately after administration (up to ~60%). On the other hand, this type of cell implantation is not physiological and may be of limited value in terms of further cell survival. However, myocardial injections may cause myocardial damage [60]. Data from the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) trial indicated an association between an increase in the number of endomyocardial injections to deliver therapeutic cells and a reduction in the therapeutic effect of mesenchymal cell transplantation in patients with chronic ischemic heart failure [61].
Human studies
With encouraging results of animal model studies, SPECT has been adopted to investigate biodistribution of 99mTc and 111In-labelled progenitor cells in a clinical setting.
The results of numerous important studies in humans confirm observations from animal model concerning cell retention and distribution. Table III provides the most relevant data from studies in humans in which SPECT was used [29, 34, 62–71].
Table III
SPECT evaluation of myocardial uptake for cell therapies in clinical studies of myocardial infarction
[i] 111In – indium-111, 99mTc – technetium 99m, HMPAO – hexamethylpropyleneamine oxime, CHF – chronic heart failure, AMI – acute myocardial infarction, BM-MSCs – bone marrow mesenchymal stem cells, PB-MSCs – peripheral blood mononuclear cells, BM-MNCs – bone marrow mononuclear cells, WJMSCs – Wharton’s jelly mesenchymal stem cells, ic – intracoronary, LAD – left anterior descending artery, IRCV – interstitial retrograde coronary venous, OTW – over-the-wire (stop-flow technique), PC – perfusion catheter (perfusion technique).
Work from our group, using SPECT, has addressed several critical questions in cardiac regenerative medicine including optimizing transcoronary cell delivery, determination of the zone(s) of myocardial cell uptake, and late functional improvement in relation to the magnitude of cell uptake [29, 32, 72, 73].
Caveliers et al. investigated homing of 111In-oxine-labeled peripheral blood stem cells in chronic ischemic heart disease conditions. The cells were infused intracoronarily through a balloon catheter with stop-flow technique. Fused 99mTc-sestamibi/111In SPECT images demonstrated the regional distribution of the transplanted cells within the no/low perfusion zone, as delineated by the flow tracer. The radioactivity retention in the heart was 6.9–8% after 1–2 h and 2.3–3.2% after 12 h [64].
Penicka et al. investigated the kinetics of myocardial engraftment of 99mTc-HMPAO-labeled bone marrow-derived mononuclear cells after intracoronary (LAD) injection in patients with acute and chronic anterior myocardial infarction. At 2 h after infusion, myocardial activity was observed in all patients with acute (range: 1.31–5.10%) and in all but 1 patient with chronic infarction (range: 1.10–3.0%). At 20 h, myocardial engraftment was noted only in 3 patients with acute IM [65].
Karpov et al. performed a randomized controlled study including 44 patients with acute myocardial infarction. It was found that intracoronary injection of bone marrow mononuclear cells is safe, ensures fixation of the injected cells in the myocardium, reduces blood levels of IL-1β and TNF-α, increases the content of insulin-like growth factor, and does not provoke malignant arrhythmias [66].
Goussetis et al. examined biodistribution of CD133+ and CD133-CD34+ 99mTc-hexamethylpropylenamineoxime-labeled selected autologous bone marrow progenitor cells infused into the infarct-related artery in patients with chronic ischemic cardiomyopathy. One and 24 h after transplantation the radioactivity in the infarcted area was 9.2% and 6.8% respectively; the remaining activity was distributed mainly to the liver and spleen, similarly to other studies [68].
Schächinger et al. transplanted circulating proangiogenic progenitor cells labelled with 111In-oxine in patients with previous myocardial infarction and a revascularized infarct vessel at various stages after infarction (5 days to 17 years). Similarly to other studies, 1 h after cell administration, 6.9% of total radioactivity was detected in the heart, which declined to 2% after 3 to 4 days. The authors also found that average activity within the first 24 h was highest among patients with acute myocardial infarction. Moreover, proangiogenic progenitor cell homing was influenced by low viability of the infarcted myocardium and reduced coronary flow reserve [74].
Silva et al. investigated the safety and feasibility of autologous bone marrow mononuclear cell (BMMNC) transplantation in ST elevation myocardial infarction (STEMI), comparing anterograde intracoronary artery (ICA) delivery with the retrograde intracoronary vein (ICV) approach. 1% of cells were labelled with 99mTc-hexamethylpropylenamineoxime. Cell distribution was evaluated 4 and 24 h after injection. The authors observed exceptionally high retention in the intracoronary group (early 16%, late 10%) as compared to the retrograde intracoronary group (early 4%, late 3%). Early and late retention of radiolabelled cells was higher in the ICA than in the ICV group [70].
As the stop-flow technique has never been shown to be mandatory in intracoronary cell transplantation, our group focused on comparing two methods of autologous 99Tc-extametazime-labeled bone marrow CD34+ cell delivery with insight into patterns of cell retention. We found that the effectiveness of the perfusion technique (side-holed perfusion catheter, cell injections under maintained coronary flow) was not different from that seen with the over-the-wire (OTW)-balloon method (stop-flow technique, 5.0% vs. 4.9% respectively). It was also found that retention of progenitor cells occurs preferentially in the (viable) peri-infarct zone, suggesting that the infarct zone is largely inaccessible to transcoronary-administered cells [29].
Moreover, the coronary-non-occlusive method delivery of Wharton’s jelly mesenchymal stem cells (WJMSCs – combining high angiogenic and cardiogenic potential with low immunogenicity) showed a high and reproducible retention rate (30%) of 99Tc-labeled WJMSCs in the peri-infarct zone in humans after recent myocardial infarction [62].
There is a significant amount of data showing a very high level of agreement between G-SPECT and other imaging techniques when considering measurement of left ventricle volumes and ejection fraction. With the most widely used, quantitative gated SPECT (QGS, Cedars-Sinai Medical Center, Los Angeles, CA), the correlation coefficients of this tool with gold standard magnetic resonance imaging reach 0.72–0.94 for left ventricle ejection fraction, r = 0.81–0.97 for end-diastolic volume and r = 0.87–0.99 for end-systolic volume [75]. Moreover, it was demonstrated that SPECT distinguishes itself from other imaging tools with its outstanding reproducibility [76, 77]. Considering regional wall motion abnormalities, gated SPECT also showed excellent (83%) agreement with MRI [78]. Apart from volumes and ejection fraction evaluation, gated SPECT allows assessment of regional LV function. The perfusion and wall motion defects have been widely adopted as outcome parameters of the human cardiac cell therapy.
Tables IV and V provide detailed data on end-point parameters acquired by SPECT in animal [47, 79–82] and human [83–98] models of trials concerning the clinical effect of progenitor cell transplantation. In contrast to encouraging outcomes in cell therapy in animals, the results of randomized trials in humans investigating the potential effect of progenitor cells transplanted into myocardium show no or a minimal effect on cardiac function. In fact, this subtle positive effect of cells transplantation on quantitative, measurable end-point parameters (ejection fraction, myocardial perfusion, regional wall motion index) is visible only in large-scale meta-analyses [99–101].
Table IV
SPECT-tracked effect of cell transplantation on myocardium function in animal model of ischemia
[i] AMI – acute myocardial infarction, HF – heart failure, LVEF – left ventricle ejection fraction, SPECT – single-photon emission-computed tomography, PET – positron emission tomography, Echo – echocardiography, BM MNSCs – bone marrow mononuclear stem cells, BMCs – bone marrow-derived cells, CPCs – circulation progenitor cells, SSS – summed stress score, SRS – summed rest score, WJMSCs – Wharton’s jelly mesenchymal stem cells, ADRC – adipose-derived regenerative cells, Tc-technetium, Tl – thallium, MIBI – methoxyisobutylisonitrile.
Table V
SPECT-tracked effect of cell transplantation on myocardium function in clinical studies of myocardial ischemia
[i] AMI – acute myocardial infarction, HF – heart failure, LVEF – left ventricle ejection fraction, SPECT – single-photon emission-computed tomography, PET – positron emission tomography, Echo – echocardiography, BM MNSCs – bone marrow mononuclear stem cells, BMCs – bone marrow-derived cells, CPCs – circulating progenitor cells, SSS – summed stress score, SRS – summed rest score, WJMSCs – Wharton’s jelly mesenchymal stem cells, ADRC – adipose-derived regenerative cells, MIBI – methoxyisobutylisonitrile.
Thus, further optimal types of cells, ways of administration and uptake, and mechanisms of regeneration are still to be investigated [102].
Progenitor cells have been recently examined as a transfer vehicle for non-viral gene delivery systems for tissue repair and regeneration therapies. In fact, gene-corrected CD34+ stem cells have already been successfully adopted for treatment of inherited diseases – progenitor cells are harvested, transduced ex-vivo with a viral vector, and then reinfused into the patient [103]. The same technology may be used in the future for therapeutic cell modification or local gene transfer into damage tissue.
In conclusion, SPECT is a technique that offers high-sensitivity, quantitative cell tracking on top of its ability to evaluate myocardial perfusion and function on both a cross-sectional and a longitudinal basis. SPECT, with its direct relevance to routine clinical practice, plays a fundamental role in evaluation of myocardial reparation and regeneration therapies (Table VI).








