Introduction
The global prevalence of infertility ranges from 10 to 15%, making reproductive disorders a significant concern in global health [1, 2]. Infertility can be attributed to various aetiological factors. Premature ovarian insufficiency (POI) is the term used to describe the permanent decrease in ovarian function that happens in women before the age of 40 years. It is defined by decreased oestrogen production and increased gonadotropin levels, which lead to a number of symptoms [3]. The prevalence of POI is approximately 1–4%, but due to changes in social environment and increased life and work pressures, its prevalence is gradually rising with a trend towards younger ages [4]. Premature ovarian insufficiency is not merely a cause of ovarian hormone deficiency and infertility but is also linked to elevated health risks including cardiovascular disease, osteoporosis, and a certain degree of cognitive decline [5]. Furthermore, it has been reported that POI patients may experience a shortened life expectancy [6]. However, due to the high heterogeneity and multifactorial nature of POI, the underlying biological mechanisms for most cases remain to be further elucidated [7].
Sleep, an intrinsic biological rhythm of the human body, is essential for maintaining homeostasis, modulating neuroendocrine functions, and regulating energy metabolism. It represents not only the cyclical physiological and psychological phenomena but also serves as a critical nexus for recovery, information integration, memory consolidation, and physical rejuvenation [8]. In recent years, sleep disturbances have garnered considerable attention as a significant risk factor impacting public health. An increasing body of research indicates a strong correlation between reduced sleep duration and quality with the heightened incidence of chronic diseases and elevated all-cause mortality, encompassing conditions such as asthma, hypertension, and myocardial infarction [9, 10]. It is noteworthy that the escalating prevalence of infertility has paralleled the rise in sleep deprivation and disruption over recent decades [11]. Studies indicate that women in their reproductive years who suffer from sleep deficiency are at an elevated risk of menstrual irregularities and insulin resistance [12]. Additionally, detrimental sleep habits, characterised by shortened nocturnal sleep duration, inconsistent sleep-wake rhythms, and inadequate amounts of sleep, can adversely affect antral follicle count, maturation, and fertilisation potential [13]. Furthermore, both insufficient and excessive sleep durations have been correlated with reduced likelihood of clinical pregnancy in women [14]. Disruptions in sleep patterns are prevalent among patients with POI, with the majority experiencing diminished sleep quality and increased susceptibility to fatigue relative to age-matched healthy controls [15]. Studies have implicated work-related and familial stressors, as well as enduring adverse life events preceding the diagnosis of POI, as significant contributors to sleep-related issues that can adversely impact health [16]. Although clinical observations suggest a correlation between sleep disturbances and POI, the nature of this association remains ambiguous due to potential confounding variables and the possibility of reverse causality.
Well-conducted randomised controlled trials are frequently regarded as the gold standard for determining causation. However, they are often resource-intensive and may face implementation challenges due to ethical considerations and budgetary limitations. Mendelian randomisation (MR) offers a compelling alternative for investigating causality, utilising single nucleotide polymorphisms (SNPs) correlated with exposure variables as instrumental variables (IVs) [17]. This approach leverages the random allocation of alleles at conception and their constancy throughout life to mitigate confounding influences and preclude bias from reverse causality, thereby enhancing the reliability of causal inferences beyond those achievable by conventional observational research [18, 19]. Given its unique advantages, MR has come out as a viable technique for evaluating the connections between numerous exposures and reproductive health outcomes [20, 21]. In this study, we will employ MR analysis on genome-wide association study (GWAS) data of sleep traits and POI to elucidate the underlying genetic causality, thereby informing future preventative and therapeutic strategies.
Methods
research design
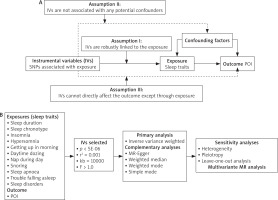
As depicted in Figure 1, we conducted both two-sample and multivariate MR analysis. The two-sample MR analysis was used to look at the association among 11 sleep traits and POI. The study cohort was restricted to individuals of European descent, thereby mitigating potential biases attributable to population stratification. The multivariate MR analysis facilitated the estimation of the causal effects of multiple risk factors on POI susceptibility by integrating all relevant exposures within a single model framework.
Data sources
Identify eligible GWAS datasets for both exposure and outcome variables, encompassing resources from the UK Biobank, Neale Lab, IEU open GWAS, and FinnGen. Given that this investigation represents a secondary analysis of publicly available datasets, no further ethical approval was necessary.
Eleven sleep-related traits, encompassing sleep duration, sleep chronotype, insomnia, hypersomnia, getting up in the morning, daytime dozing, nap during day, sleep apnoea, snoring, trouble falling asleep, and sleep disorders, were extracted from GWAS datasets to serve as exposure variables. Premature ovarian insufficiency was designated as the outcome measure. In this investigation, the corresponding phenotypic code referenced was ‘finn-b-E4_OVARFAIL’. An overview of the database is presented in Table 1.
Table 1
Summary of genome-wide association study data
The selection of instrumental variables
To ensure the precision and robustness of the MR analysis, a stringent IV selection process was applied to the 11 sleep traits. Adhering to the 3 fundamental assumptions underlying MR, we utilised the genome-wide significance threshold for SNPs (p < 5 × 10–6) and linkage disequilibrium criteria (r2 < 0.001, 10000 kb) to ascertain the independence and relevance of the SNPs. Additionally, we employed F-statistics to assess the efficacy of the IVs, excluding those with F-statistics below 10 to mitigate the potential bias associated with weak instruments [22, 23].
Mendelian randomisation analysis
In the two-sample MR analysis, we employed the inverse variance weighted (IVW) method as the primary analytical approach. The inverse variance weighted method is widely recognised as the gold standard for evaluating causality [24]. To bolster our analysis, we additionally incorporated the MR-Egger, weighted median, simple median, and weighted mode methods. The MR-Egger method ascertains the causal effect by leveraging the slope coefficient derived from Egger regression, offering a more robust causal estimation, even in scenarios where some IVs may not be valid. The weighted median method can potentially exclude up to 50% of non-informative IVs, thereby enhancing the precision of thte analysis. The weighted mode method approach necessitates the identification of multiple variables to serve as an effective instrument for detecting the same causal effect. The simple median method categorises SNPs with analogous values into clusters and employs the cluster with the highest frequency of SNPs to assess causal relationships [25–27].
Building upon the two-sample MR analysis, the multivariate MR approach enables the estimation of the causal effects of multiple risk factors on the risk of POI by integrating all relevant exposures within a unified model framework [28]. We combined the significant exposure factors in the two-sample MR analysis. After excluding repeated SNPs, we obtained the effect of each SNP and the corresponding standard error from the exposure and results. In multivariate MR analysis, both IVW and MR-Egger methods based on weighted linear regression are used to infer causality.
Sensitivity analysis
Cochran’s Q statistical analysis was conducted to assess heterogeneity among the studies. The resulting p-value from Cochran’s Q test exceeded 0.05, indicating that there was no significant heterogeneity [29]. The MR-Egger intercept was employed to scrutinizse the potential pleiotropic effects. A p-value greater than 0.05 indicated no evidence of pleiotropy [30]. The leave-one-out method was employed for conducting sensitivity analyses, wherein the MR effect estimation was iteratively recalculated. By sequentially excluding a single genetic variant per iteration, the analysis assessed the individual contribution of each variant to the composite estimation, identified potential outliers, and affirmed the robustness of the aggregate effect [31]. The funnel plot was used to detect result bias.
Statistical analysis
All Mendelian randomisation analyses were conducted utilising R software, version 4.4.1. The R packages used included ‘MRInstruments’, ‘MRPRESSO’, ‘TwoSampleMR’, ‘MVMR’, and ‘MendelianRandomization’. Statistical significance was defined as a p-value of less than 0.05 for all analyses.
Result
Two-sample Mendelian randomisation analysis
This study encompassed 11 sleep-related traits as exposures, with each IV exhibiting an F-statistic exceeding the threshold of 10. As delineated in Figure 2 a positive correlation was observed between sleep duration and POI, whereas an inverse relationship was noted between sleep chronotype and POI.
Fig. 2
Two-sample Mendelian randomisation for different exposures with premature ovarian insufficiency
MR-Egger – mendelian randomization-Egger, nSNP – number of single nucleotide polymorphism, SM – simple median, WM – weighted mode, WME – weighted median


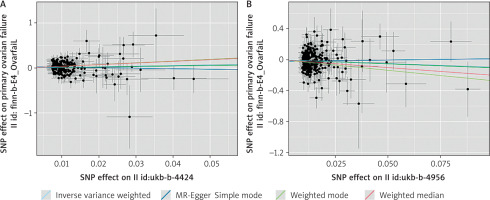
In the association study between sleep duration and POI, 8 palindrome SNPs (rs11043328, rs12670234, rs17732997, rs2186122, rs2249966, rs269054, rs7943526, rs8015629) were excluded before MR analysis, and 215 SNPs were finally included. The inverse variance weighted method found a positive connection between sleep duration and POI (β = 1.464, OR = 4.323, 95% CI: 1.093–17.112, p = 0.037), showing that longer sleep duration reduced the risk of POI.
In the association study between sleep chronotype and POI, 14 palindrome SNPs (rs1000665, rs1348990, rs1837446, rs2304467, rs3796618, rs4533855, rs4595586, rs4729854, rs4784655, rs7210415, rs7586062, rs812925, rs848552, rs9962650) were excluded before MR analysis. Finally, 316 SNPs were included. The inverse variance weighted method found a negative connection between sleep chronotype and POI (β = –0.915, OR = 0.400, 95% CI: 0.173–0.925, p = 0.032 ), suggesting that preferring morning sleep may lower the incidence of POI. Figure 3 shows the scatter plot of the MR analysis of 2 sleep traits.
Sensitivity analysis
Cochran’s Q test results indicated no significant heterogeneity within the MR analysis outcomes for sleep duration and sleep chronotype. The p-values derived from the MR-Egger regression intercepts consistently exceeded 0.05, suggesting the absence of horizontal pleiotropy (Table 2). The leave-one-out method demonstrated that the exclusion of individual SNPs did not substantially influence the estimation of causal relationships, thereby confirming the robustness of the MR analysis findings (Fig. 4). Additionally, the funnel plot depicting the distribution of causal effects exhibited fundamental symmetry, with no evident signs of bias (Fig. 5).
Multivariate Mendelian randomisation analysis
Two POI-related sleep traits were selected for multivariate MR analysis to correct the effects of multiple sleep traits on the outcome. The results showed that when sleep duration (OR = 0.362, 95% CI: 0.038–3.385, p = 0.373) and sleep chronotype (OR = 0.502, 95% CI: 0.168–1.495, p = 0.216) were activated at the same time, there was no common effect on POI.
Discussion
Leveraging an extensive array of openly accessible genetic datasets, we delineated the causal nexus between 11 sleep-related traits and POI. To our knowledge, this is the first MR analysis to investigate the causal relationship between a range of sleep traits and POI. Our findings from the two-sample MR analysis indicate a significant causal nexus between sleep duration and chronotype with POI. Conversely, the multivariate MR analysis did not discern a concerted effect of these sleep traits on POI.
Research has indicated that women with POI typically exhibit poorer sleep quality, extended sleep durations, and elevated fatigue indices relative to their counterparts with normal ovarian function [32]. Moreover, the prevalence of inadequate sleep duration and sleep disturbances is higher in infertile women compared to fertile women, with a significant increase in infertility risk observed in women whose sleep duration is confined to ≤ 7 hours [33]. The aforementioned findings align with our MR analysis, substantiating the observed relationship between sleep duration with POI. Drawing from the clinical profiles and MR-derived insights of POI patients, we postulate that inadequate sleep duration could be a risk factor for POI, with sufficient sleep potentially mitigating disease risk. The interplay between circadian rhythms and sleep homeostasis is crucial for modulating sleep duration and associated physiological mechanisms. Chronic sleep deficiency and deprivation are implicated in the disruption of circadian rhythms [34, 35]. Physiologically, folliculogenesis exhibits distinct circadian patterns, synchronised with photoperiod and endocrine cues [36, 37]. Key regulators of female reproductive function, such as sex steroid hormones, gonadotropins, and sex hormone-binding globulin (SHBG), are subject to the influence of endogenous circadian rhythms, with a potentially heightened influence during the follicular phase [38, 39]. Circadian clock genes, notably BMAL1, are pivotal in modulating reproductive functions. The rhythmic expression of BMAL1 in ovarian granulosa and theca cells significantly impacts the synthesis of ovarian steroid hormones and the ovulatory process. Conditional deletion or aberrant transcription of the BMAL1 gene in theca cells alters the mRNA expression pattern of the pituitary luteinising hormone (LH) receptor, leading to reduced ovarian sensitivity to LH and compromised ovulation efficacy [40, 41].
Furthermore, the activation of primordial follicles during the female reproductive cycle is constrained, and disruptions to this process can precipitate aberrant follicular development. Excessive primordial follicle activation may diminish the ovarian reserve pool [42]. Premature ovarian insufficiency often arises from an early depletion of the follicular reserve and heightened follicular atresia, culminating in impaired oocyte and follicular development alongside ovarian dysfunction. Sleep deprivation may deplete follicular reserves, potentially by accelerating the activation and atresia of primordial follicles [43]. The study also highlighted that sleep insufficiency can induce alterations in the gut microbiota of adolescent women, potentially impacting the systemic metabolism of the nicotinamide adenine dinucleotide (NAD+) pathway, thereby compromising the mitochondrial energy metabolism in ovarian oocytes and leading to reduced POI and oocyte meiotic competence [43].
Our MR analysis found a substantial negative causal link between sleep chronotype and the probability of POI, suggesting that individuals with a propensity for early morning sleep patterns may lower their risk of POI. Analogous to various biological processes, sleep patterns are intricately linked to immune responses, inflammatory processes, and fluctuations in circulating immune cell counts [44]. Cytokines such as interleukin-1β, interleukin-6 (IL-6), and tumour necrosis factor-α (TNF-α) play pivotal roles in chronic inflammation. Zhai et al. [45] reported elevated levels of these inflammatory markers in young individuals with late sleep chronotypes. Furthermore, Ma et al. [46] highlighted that during pregnancy, sleep insufficiency, particularly late sleep onset and snoring, may exacerbate systemic chronic inflammation. Chronic inflammation, a pervasive condition over the long term, can result in sustained elevated levels of inflammatory mediators, including IL-6 and TNF-α, potentially leading to irreversible pathological alterations within the ovarian tissue of females [47]. In POI patients, the engulfment of senescent cells via phagocytosis and the extensive apoptosis of ovarian macrophages are frequently observed phenomena, accompanied by an upregulation of pro-inflammatory cytokines and a downregulation of anti-inflammatory cytokines, as seen in POI animal models [48, 49]. Furthermore, chronic inflammation may disrupt the homeostatic mechanisms of the hypothalamic-pituitary-ovarian axis, precipitating hormonal imbalances that can adversely impact ovarian function [50].
In addition, sleep chronotype is likely to influence the secretion of sex hormones. Ren et al. [51] demonstrated that sleep chronotype significantly impacts not only the secretion of female testosterone but also exhibits a substantial causal link with oestrogen secretion, with early morning sleep patterns being more favourable for oestrogen production. Similarly, Peplonska et al. [52] reported an inverse correlation between the frequency of night shifts among premenopausal nurses and circulating oestrogen levels. Given that a primary characteristic of POI is the erratic decline in oestrogen levels [53], it is plausible to posit that sleep chronotype may indirectly contribute to the pathogenesis of POI through the modulation of oestrogen levels.
Additionally, we employed a multivariate MR analysis, which demonstrated no detectable shared causal influence of the 2 sleep traits on the probability of POI. This investigation utilised a diverse array of MR methodologies to furnish compelling evidence supporting the causal nexus among sleep traits with POI risk. However, it is important to recognise the study’s shortcomings: the absence of individual-level data precludes a more granular stratified analysis, thereby constraining an in-depth exploration of the impact of sleep traits on discrete populations. The research’s primary reliance on a European population database may also restrict the generalisability of the findings across diverse ethnicities. Ultimately, to enhance the comprehensive assessment of the correlation between sleep traits and POI, a more lenient threshold was applied in our analysis, which could potentially elevate the likelihood of false-positive outcomes.
Conclusions
We discerned a positive correlation between sleep duration and the risk of POI, while an inverse relationship was observed with sleep chronotype. However, no shared causal influence of these sleep traits on POI risk was identified. Subsequent research endeavours should explore the potential of enhancing sleep patterns through behavioural interventions and pharmacological treatments to mitigate the risk of POI. The development of these preventive and therapeutic strategies merits further investigation within clinical settings.