Introduction
Cluster headache (CH) is a primary headache and is characterized by recurrent attacks of very severe unilateral pain located in and around the orbit that last 15 min to 3 h. It occurs at a frequency ranging from once every other day to 8 times a day. The severe pain is usually accompanied with ipsilateral cranial autonomic symptoms related to the eyes and nose that present as conjunctival flushing, lacrimation, eyelid edema, nasal and/or sinus congestion with rhinorrhea and redness of the face [1]. CH is considered to be one of the worst pains that humans can experience. The intensity of pain is so high that it can induce suicidal thoughts, and so CH has been named “suicidal headache” [1]. In accordance with the International Classification of Headache Disorder (ICHD-3), CH is classified into episodic CH and chronic CH [2]. Episodic CH is characterized by regular headache cluster periods that vary in duration from 2 weeks to 3 months followed by a relief period that last longer than 2 weeks, while chronic CH lacks a subsequent relief phase lasting longer than 14 days for a minimum of 1 year [2].
So far, the pathophysiology of CH remains unclear; however, the sphenopalatine ganglion (SPG) has been considered to play an important role in CH [3, 4]. For patients refractory to traditional pharmacologic treatment, SPG targeted invasive treatments, such as SPG block and SPG transection, were considered, but the treatment effects were unsatisfactory and the recurrence rates were high [5, 6].
In recent years, SPG targeted radiofrequency thermocoagulation has been reported to be effective in the treatment of CH, but it can cause variable degrees of cheek numbness and hypoesthesia of the palate in patients because the sensory roots in the SPG deriving from the sphenopalatine branch of the maxillary nerve could be irreversibly damaged at 55–70°C [5].
SPG targeted pulsed radiofrequency ablation is another procedure reported for the treatment of CH with less tissue destruction and less painful [7–9]. The disadvantages of pulsed radiofrequency ablation are that there is typically a shorter duration of relief and the procedure may need more frequent repeat treatments, which can lead to a higher cost [9, 10].
Low-temperature plasma radiofrequency ablation is a relatively novel technique with promising applications in neuropathic pain. It has been suggested to be effective for the treatment of phantom limb pain [11], trigeminal neuralgia [12] and thoracic neuropathic pain [13], among others. It uses radiofrequency energy through a saline medium to create plasma iron particles that break molecular bonds in the tissue, which causes the tissue to dissolve at relatively low temperatures. In the present study, SPG-targeted low-temperature plasma radiofrequency ablation was applied in the treatment of refractory CH. Its efficiency and safety were evaluated.
Aim
To evaluate the efficacy and safety of sphenopalatine ganglion-targeted low-temperature plasma radiofrequency ablation in the treatment of patients with refractory CH.
Material and methods
General information of patients
A retrospective study was conducted on 75 patients with CH who underwent computed tomography (CT)-guided SPG-targeted low-temperature plasma radiofrequency ablation in the Department of Pain Management, Xuanwu Hospital between January 2015 and December 2017. This study was approved by the Ethics Committee of Xuanwu Hospital. Among 75 patients 32 were male and 43 were female. The age ranged from 26 to 84 years (mean age: 48 years). The course of disease ranged from 6 months to 40 years, with mean duration of 13.50 years. 36 cases were on the left side and 39 cases were on the right side (Table I). All cases were in compliance with CH diagnostic criteria published by the International Pain Association (International Headache Society – IHS) [2], with 54 episodic CH and 21 chronic CH. All patients were refractory to conventional pharmaceutical treatments.
Procedures
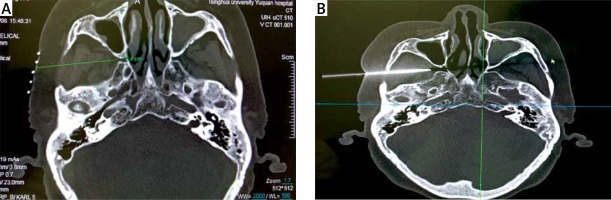
SPG-targeted low-temperature plasma radiofrequency ablation was performed under CT guidance. The patient was asked to lie supine on the CT scanner bed. A CT scan was performed to locate the pterygopalatine fossa and the entry route was marked (Photo 1 A). Under local anesthesia with 1% lidocaine, a 9.5 cm 18G puncture needle was inserted and adjusted to reach the pterygopalatine fossa under CT guidance (Photo 1 B). After the tip of the needle was optimally positioned, the needle core was replaced with the plasma ablation needle (DXR-G1100-A185; Xi’an Surgical Medical Technology Co., Ltd., Xi’an, China). To further confirm that the position was adequate, sensory stimulation was performed. The Stimuplex HNS11 Nerve Stimulator (B. Braun Co., Ltd., Melsungen, Germany) was connected to the plasma ablation needle. The nerve stimulator was turned on, adjusted to 2 Hz and 0.1 ms, and the stimulus intensity was gradually increased to 0.5 mV, adjusting the location of the plasma ablation needle until paresthesia was induced along the innervation of the nasal root. If the stimulus was felt in the hard palate (palatal nerve) or the upper tooth (maxillary nerve), the position of the puncture needle needed to be readjusted. The plasma ablation needle was disconnected from the nerve stimulator and connected to the low-temperature plasma generator (SM-D380C; Xian Surgical Medical Technology Co., Ltd., Xian, China). Under intravenous general anesthesia, ablation was performed at 45 W for 30 s, and repeated after a 30 s pause.
Data collection and follow-up
Physical examination was conducted routinely before the procedure and on day 1 and day 3 after the procedure. Follow-up was conducted routinely at 3 and 6 months, and annually after the procedure. The evaluation of pain was performed using the numeric rating scale (NRS) with 0 as no pain and 10 as intolerable pain. The numeric rating scores were recorded on the day before the procedure and 1 and 3 days, 3 and 6 months and annually after the procedure. The efficacy was evaluated based on NRS scores. Complete relief (CR) was defined as no pain (0 points on the NRS), no parasympathetic symptoms without pharmacologic treatment. Partial relief (PR) was defined as a decreased intensity of the attacks resulting in a reduced medication need. No relief was defined as the pain level remaining the same as before the procedure. Effective relief was defined as complete relief or partial relief.
For cheek numbness, the patients were asked whether they had a feeling of numbness. Hypoesthesia during hospitalization was tested through physical examination. A cotton swab was used to lightly touch patients’ cheeks on both sides and the patients were asked whether the cheeks on both sides had the same feeling. For muscle weakness or atrophy, the patients were checked and asked whether their faces were symmetrical and whether there was muscle weakness when chewing and biting.
Results
Efficacy in pain relief and pain recurrence
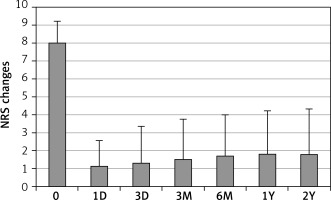
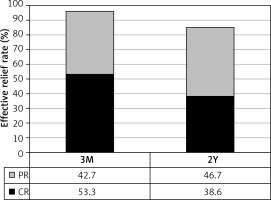
Obvious pain relief was observed after the procedure. NRS scores dramatically decreased from 7.97 ±1.25 before the procedure to 1.14 ±1.42, 1.30 ±2.05, 1.50 ±2.25, 1.71 ±2.30, 1.80 ±2.42 and 1.83 ±2.53 at 1 and 3 days, 3 and 6 months, 1 and 2 years after the procedure, respectively (Figure 1). There was a significant difference between NRS score before the procedure and NRS score at any time point after the procedure (p < 0.001 in all). Since the episodic CH patients had different time of pain free interval between pain cluster periods, the effective relief at 3 months after the procedure was considered as the initial treatment outcome. The effective relief rate at 3 months after the procedure was 96% with 40 (53.3%) patients achieving complete relief with no any pain and 32 (42.7%) patients obtaining partial relief with less severe pain (NRS scores, mostly 2–4) (Figure 2). Three (4%) patients showed no relief. These three no pain relief cases were considered as invalid ablation. All patients received at least 2 years of follow-up. The effective relief rate at 2 years after the procedure remained as high as 85.3% with 29 (38.6%) obtaining complete relief and 35 (46.7%) partial relief (Figure 2). Eleven (14.7%) patients showed no relief including 3 previous invalid ablation cases and 8 (10.6%) recurrent cases. The recurrence occurred at different time points after the procedure including 6 months (3 patients), 1 year (3 patients) and 2 years (2 patients). Among 75 CH patients, 54 had episodic pain and 21 had chronic clustered pain. The effective relief rate at 2 years after the procedure in chronic clustered cases was 85.7% with 8 (38.1%) cases of complete relief, 10 (47.6%) of partial relief and 3 (14.2%) of no relief. This outcome was not significantly different compared to the total group. Therefore SPG low-temperature plasma radiofrequency ablation was equally effective for both episodic and chronic clustered CH.
Complications
Twenty-nine (38.7%) patients had mild and moderate degrees of facial numbness immediately after ablation. Facial numbness gradually improved and disappeared within 6 months with duration of 10.5 ±7.48 weeks. Additional post-procedure complications including mild masseter weakness (2 cases, 2.7%), cheek hypoesthesia (9 cases, 12%) and cheek hematoma (2 cases, 2.7%) were observed and all disappeared within 3 months.
Discussion
The sphenopalatine ganglion has been proven to play an important role in CH. For patients who have not responded to drug treatment or cannot tolerate the treatment-related side effects, SPG-targeted invasive treatments are taken into consideration. In recent years, it has been reported that SPG radiofrequency ablations, both thermal and pulsed, are relatively quick, cost-effective and safe procedures for treating CH. Radiofrequency thermocoagulation (RFTA), also known as radiofrequency neurotomy, is a method used to destroy painful nerves with 70°C to 90°C heat. The RFTA device uses high frequency (ranges: 300–500 kHz) to create charged molecular oscillation which generates heat by the friction of ions and radio waves. In conventional RFTA, the special RFTA needle generates a 5–15 mm electric field that ultimately increases the temperature of the affected tissue. This temperature produces local tissue damage and loss of myelinated fibers. When the needle tip heats to 80°C for 60 to 90 s, it reliably produces an 8–10 mm affected area. RFTA is effective for pain relief, but it can cause variable degrees of cheek numbness or dysesthesia of the palate, maxilla, or post pharynx in patients because the sensory roots in the SPG coming from the sphenopalatine branch of the maxillary nerve could be irreversibly damaged by heat. Pulsed radiofrequency ablation (PRFA) is another radiofrequency technique. It is performed with similar short 20-ms pulses every 0.5 s, which allows time for the tissue to cool and does not exceed the target temperature of 42°C. The advantage of PRFA is that there is less tissue destruction and that it is less painful. The disadvantages of PRFA are that there is typically a shorter duration of relief and the patients may need more frequent repeat treatments, which can lead to a high cost [7–9].
Low-temperature plasma radiofrequency ablation is a relatively novel minimally invasive technique with promising applications in neuropathic pain [11–13]. Its working principle is to make the local tissue around the electrode tip form a plasma field by 100 kHz radio frequency energy; a thin layer plasma of 50–100 ¼m with a large number of ion particles carrying enough kinetic energy is formed, ablating target tissues by cutting off organic molecular bonds in tissues at low temperatures of about 40–50°C. Therefore, the principle action of plasma radiofrequency ablation is based on molecular dissociation at low temperature rather than thermal damage as in radiofrequency thermocoagulation [14, 15].
Salgado-Lopez et al. [7] published their results on the safety and efficacy of SPG radiofrequency ablation (both RFTA and PRFA) for treating chronic CH. They followed 37 patients for a mean follow-up of 68.1 months (range: 15–148 months). Five (13.5%) of the patients experienced complete clinical relief of both pain and parasympathetic symptoms, 21 (56.8%) patients had partial and transient relief, and 11 (29.7%) patients did not improve. Chen et al. [9] reported their results of SPG pulsed radiofrequency (PRFA) in the treatment of CH. In total 59 patients underwent 106 PRFA procedures within an observational period of 24 months (range: 3–108 months). Effective remission at 30 days after the PRFA procedure was observed in 95.6% and 64.3% of patients with refractory episodic and chronic CH, respectively. However, high recurrence was observed and repeated PRFA procedures were performed for recurrent patients.
In this study, CT-guided SPG low-temperature plasma radiofrequency ablation was applied in the treatment of 75 patients with refractory CH. Obvious pain relief was observed immediately after the procedure. The effective relief rate at 3 months after the procedure was 96%. Forty (53.3%) patients achieved complete relief; 32 (42.7%) patients obtained partial relief and 3 (4%) patients showed no relief. These three no pain relief cases were considered as invalid ablation. All patients completed at least 2 years of follow-up. The effective relief rate at 2 years after the procedure remained as high as 85.3% with 29 (38.6%) patients achieving complete relief and 35 (46.7%) patients obtaining partial relief. Eleven (14.7%) patients showed no relief including 3 previous invalid cases and 8 (10.6%) recurrent cases. These results indicated that SPG-targeted low-temperature plasma radiofrequency ablation is effective for the treatment of refractory CH.
It has been reported that chronic CH showed more resistance to the treatment of pulsed radiofrequency ablation compared to episodic CH [7–9]. In this study, among 75 CH patients, 21 cases were chronic CH. The effective relief rate at 2 years after the procedure for 21 chronic CH was 85.7% with 8 (38.1%) complete relief, 10 (47.6%) partial relief and 3 (14.2%) no relief. This result is similar to the outcome of total group. Our data demonstrated that SPG-targeted low-temperature plasma radiofrequency ablation was equally effective for both episodic and chronic clustered CH.
The low-temperature plasma radiofrequency ablation procedure causes fewer complications compared to radiofrequency thermocoagulation because it uses plasma ion particles to cut off organic molecular bonds in tissues at low temperatures of about 40–50°C rather than 70°C to 90°C as in radiofrequency thermocoagulation. In the comparative study of Gasserian ganglion-targeted low-temperature plasma radiofrequency ablation vs. radiofrequency thermocoagulation in the treatment of trigeminal neuralgia performed in our department, facial numbness in the plasma group (44%) was dramatically lower than that in the thermocoagulation group (70.9%) [12]. In this study, 29 (38.7%) patients had mild to moderate degrees of facial numbness immediately after ablation. Facial numbness gradually improved and disappeared within 6 months with duration of 10.5 ±7.48 weeks. Additional post-procedure complications included masseter weakness (2 cases, 2.7%), cheek hypoesthesia (9 cases, 12%) and cheek hematoma (2 cases, 2.7%). All these complications were mild and disappeared within 3 months. No severe complications were observed in this study.