Summary
Expression of haematopoietic stem and progenitor cells in patients scheduled for coronary artery bypass surgery (CABG) was not related by cardiopulmonary bypass. The inflammatory reaction was associated with preoperative trauma and diabetes is thought to be a down-regulating factor for mesenchymal stem cells in that response.
Introduction
Currently, the term “stem cell” is understood as cells characterized by the same ability of self-renewal, asymmetric division, and the possibility to differentiate into different types of mature cells [1]. In humans about 260 types of cells have been distinguished. Based on their phenotypic features about 30 different subpopulations of stem and progenitor cells have been identified among them [2]. In cellular therapy using stem cells in the field of cardiology and cardiac surgery, the greatest experience exists in studies using an unselected population of mononuclear bone marrow cells (both haematopoietic and mesenchymal lineage) [3]. Expression of haematopoietic stem and progenitor cells occurs as a result of an inflammatory reaction caused by a damaged organ [1]. Patients undergoing coronary artery bypass surgery are exposed to an inflammatory reaction, which may be a factor in the mobilization of progenitor cells [4]. The extracorporeal circulation is one of the forms of the generalized inflammatory reaction. It is used, inter alia, to perform coronary artery bypass surgery. This condition observed in cardiac surgery may result from many processes such as blood contact with a foreign, non-physiological surface of the device, tissue ischaemia, reperfusion injury, and endotoxaemia. All these processes carry a humoral and cellular response that accompanies an inflammatory reaction [5]. Intraoperative pharmacotherapy, patient temperature during surgery, type of oxygenator, drains, or fluids that fill the oxygenator system have a significant impact on the size, duration, and severity of the inflammatory reaction [6]. The dynamics and intensity of this process depend significantly on the technique of extracorporeal circulation [5].
Aim
In our study we aimed to determine the impact of the stress reaction during cardiopulmonary bypass (CPB) on mobilization of stem and progenitor cells in patients scheduled for a coronary artery bypass surgery (CABG) procedure, and its corelation with clinical status.
Material and methods
The final study group comprised of 20 patients scheduled for elective CABG recruited in the Department of Cardiac Surgery at the Medical University of Silesia in Katowice. The study protocol conforms to the ethical Declaration of Helsinki guidelines, and it was approved by the Medical University of Silesia Ethics Committee (KNW/0022/KB1/65/10). All patients signed a written informed consent form. Inclusion criteria where: age 18–75 and stabile multivessel coronary artery disease. Exclusion criteria where as follows: myocardial infarction up to 3 months before cardiac surgery, previous cardiac revascularization, any other concomitant cardiac surgery procedure, kidney failure, cancer, or autoimmune disease. The extracorporeal circulation was performed according to the normovolaemic haemodilution protocol. The patients enrolled in the study received 10 ml EDTA blood at 5 time points: (1) 2 h before the operation begins; (2) intraoperatively before connecting the extracorporeal circulation; (3) intraoperatively after disconnection of extracorporeal circulation; (4) 24 h after cardiac surgery; and (5) 6 days after cardiac surgery.
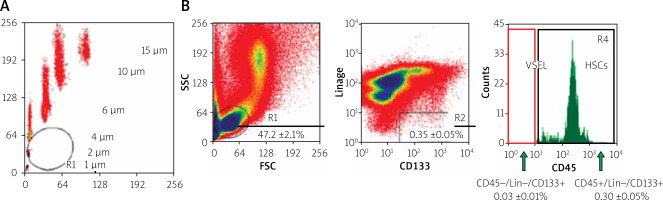
The next step was cell identification. Then the blood was lysed (BD lysing buffer, BD Biosciences, San Jose, CA) for 15 min at room temperature, followed by a suspension of cells rinsed twice in PBS. After lysis of erythrocytes and enzymatic digestion of tissues, the cells were labelled with monoclonal antibodies: linear markers (CD2 clone RPA-2.10, CD3 clone UCHT1, CD14 clone M5E2, CD66b clone G10F5, CD24 clone ML5, CD56 clone NCAM16.2, CD16 clone 3G8, CD19 clone HIB19, CD235a clone GA-R2) FITC conjugated, CD45 (clone HI30) coupled to PE, CXCR4 (clone 12G5), CD34 (clone 581), and CD133 (CD133/1) APC-coupled (BD Pharmingen, MiltenyBiotec), for 30 min on ice. Populations of CXCR4+ lin-CD45-, CD34+ lin-CD45-, and CD133+ lin-CD45- were sorted from the nuclear nucleus suspension by intravital multiparameter sterile cell sorting using a sorting cytometer. Immunophenotyping was assessed using a flow cytometer (BD Biosciences, San Jose, CA; Cell Quest software). The isolation method based on cell size (Figure 1 A) and expression of surface (Figure 1 B) markers was used.
Analyses of immune-labelled cells were carried out on an Image Stream (IS) system according to the guidelines [7]. After cell lysis, fixation, and permeabilization, the cells were stained with the following antibodies: PE anti-Oct-4 (Chemicon, USA) secondary antibody (BioLegend, USA), anti-CD45 (FITC; clone 30-F11), and antibodies against markers: ropes, CD34, CD45, and CXCR4. Granulocytes and erythrocytes were stained for the presence of CD66b markers (FITC, clone G10F5) and 235a (FITC, clone GA-R2). Cell nuclei for visualization were stained with a 10 mM Hoechst 33342 dye solution for 10 min before analysis. Analysis of samples was performed using ImageStream.
In the next stage of the analysis, the number of cells of individual lines was found at defined time points relative to the ‘0’ point, and the lines were divided into groups; thanks to this, we have an image of how the number (‘mobilization’) of cells changed over time relative to the ‘0’ point. Spearman correlation was used to determine the correlation coefficient and its statistical significance system (Beckton Dickinson, USA).
The distribution of most variables differed significantly from the normal distribution (p < 0.05 in the Shapiro-Wilk test). Differences between groups meeting the normal distribution criteria were examined using a one-way analysis of variance for repeated measurements, i.e. an ANOVA test for dependent groups. In contrast, variables that do not meet the above criteria were used to analyse the variance of repeated measurements for ranks with a post-hoc analysis of the Dunn test. Differences and dependencies for which the p-value was < 0.05 were considered statistically significant.
Results
Twenty patients (17 males) with stabile coronary artery disease were included in the study group. All patients had preserved left ventricle fraction without any other indications for cardiac surgery. In all patients CPB was performed where X-clamp time, CPB time, troponin T (hs) level, and haemodilution (pre and post haematocrit level) were not significant. Patients’ characteristics are presented in Table I. The following population of stem cells in the peripheral blood of patients was identified by isolation based on cell size and expression of surface markers using flow cytometry with the ImageStream system: HSC (Figure 2 A), MSC (Figure 2 B), EPCs (Figure 2 C), and VSEL (Figure 2 D). Each stem cell population was identified based on the expression of lineage markers (or lack of) characteristic for the population of system-labelled triplet antibodies (Table II). In this method, more than one subpopulation was generated in each cell population. Subpopulations were determined as a result of analysing data provided by flow cytometry and the ImageStream system.
Table I
Patients’ characteristics (n = 20, male 17, female 3)
Table II
Linear markers of stem and progenitor cells
| HSC | VSEL | MSC | EPC |
|---|---|---|---|
| Lin– | Lin- | Stro-1+ | KDR+ |
| CD45+ | CD45– | CD45– | CD31+ |
| CD34+ | CD34+ | CD34– | CD34+/– |
| CD133+ | CD133+ | CD105+ | CD133+ |
| CD90+ |
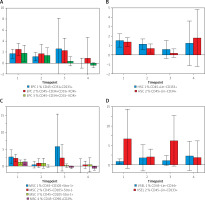
The kinetic release in both immature EPC subpopulations and their mature forms were before connecting the extracorporeal circulation and decreased, until it was completely stopped 24 h after cardiac surgery. On the 6th day, their number falls below the baseline values (p > 0.05) (Figure 3 A). In both subpopulations of HSC1 and HSC2, the same release profile is seen (p > 0.05) (Figure 3 B). Depending on the degree of differentiation, the release kinetics of MSC have the same profile as EPC or HSC. Unselected populations undergo a release process before the lung-heart apparatus is connected, and extracorporeal circulation does not intensify this reaction (p > 0.05) (Figure 3 C).
VSELS are represented by 2 cell phenotypes (VSEL1 and VSEL2), and the mobilization process did not meet the level of significance either (p > 0.05) (Figure 3 D).
The release of human stem and progenitor cells into the blood serum triggered by CPB did not occur in any of the 4 tested populations cells lines. However, some release process takes place when the cardiac surgery has already begun, but the patient has not yet been connected to the CPB. This may be due to the patient’s strong stress response before surgery and trauma associated with the tissue and bone continuity disruption.
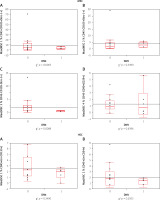
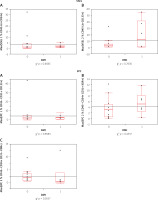
At the last stage, the distribution number of cells of individual lines was analysed, statistically testing the differences in the medians of the number of cells in the subgroups with the following clinical data of patients: arterial hypertension vs. no hypertension, dyslipidaemia vs. no dyslipidaemia, smokers vs. non-smokers, and coronary heart disease burden (data not shown). In all the above-mentioned clinical relationships, we found no statistically significant differences. Only patients with type 2 diabetes vs. no diabetes presented lower cell release (Figure 4). A lower release trend was significant in MSC (MSC3, p = 0.01) and approached the level of significance in EPC (EPC3, p = 0.05).
Finally, correlations between two selected cell line parameters were evaluated and combined with variable clinical data such as age and troponin showing the lack of correlation between individual populations (p > 0.05, data not shown). No significance in troponin level confirmed the lack of perioperative myocardial infarctions during cardiac surgery, which could overlap inflammatory processes.
In the demographic data, we only found a negative correlation with age, occurring in the EPC population (EPC1, p = 0.04) (EPC2, p = 0.02).
Discussion
Our study involved patients scheduled for an elective CABG procedure identified in peripheral blood following stem and progenitor cell lines EPC, HSC, MSC, and VSEL by superficial linage markers in IS. Mobilization of stem and progenitor cells induced by CPB did not occur in any of the isolated cell lines (p > 0.05). The profile of cell release seems to coincide with the perioperative inflammatory response. The expression profile of stem and progenitor cells seems to be related with the inflammatory reaction associated with traumatic stress in all cell lines. According to age, negative correlation was documented in EPC. When clinical comorbidities were analysed, type 2 diabetes mellitus was to down-regulate the trend of MSC mobilization.
Endothelial progenitor cells were initially considered as a group of cells mobilized from the bone marrow, which participate in the generation and repair of the vascular endothelium [8]. EPCs have recently been regarded as a heterogeneous population of cells in different stages of maturation, with different origins and several residing sites, such as the spleen, vascular endothelium, and adventitia [9]. Hill et al. found EPCs in peripheral blood when developing a colony-forming assay based on mononuclear cell culture on fibronectin-coated plates. After coronary artery surgery EPC mobilization was documented on-pump and off-pump [10]. Also, after heart valve surgery expression of previous mentioned cells were identified [11]. Trying to answer the question of what the trigger of this mobilization was, our study revealed CPB as a nonimportant stress trigger for EPC expression. Our second finding confirms the findings of Altabas et al., who studied the ability of EPCs, and they concluded that it depends on their number and functionality, which was impaired by diabetes mellitus [12]. Age is undoubtedly the next factor with which the number of EPCs decreases [13–15]. Our research revealed negative correlation with age in 2 subpopulations of EPCs.
Peripheral blood is not the classical source of haematopoietic stem cells in the case of bone marrow [16]. Although peripheral blood is a preferable source of transplantation HSCs, because it is safe, and the desired cell number can be achieved easily [17], mobilization of HSC after on-pump CABG did not occur in the presented study. However, this study was the first to investigate the relationship between CPB and stem release.
MSCs have a homing ability, meaning that they can migrate into injured sites, and they possess the capacity to differentiate into local components of injured sites. Their pluripotency and paracrine ability to extract chemokines, cytokines, and growth factors that help in tissue regeneration make MSCs the most promising regeneration cells nowadays [18]. The classic source of MSC is still bone marrow, but adipose tissue is easy and safe, and almost 100% of allogenic cells derived from adipose tissue are viable [19]. The presence of these cells has been confirmed in almost every human organ [20–22]. A few years ago there was still controversy about whether MSC can be detected in the blood circulation in humans, and Zvaifler et al. tried to isolate stem cells from sterile blood packages obtained from blood transfusion [23]. Today we know that peripheral blood is a safe, available, and minimally invasive source for isolation of MSCs [24–27]. Cardiopulmonary bypass as an inflammatory reaction releasing MSCs into peripheral blood was not confirmed in our study. But we confirmed the findings Tsukada et al. [28] of lower MSC levels in diabetes mellitus vs. non-diabetic patients. Adiponectin levels seem to play an interesting role in that mechanism [29]. Lower MSC levels in patients with diabetes mellitus were also documented in adipose tissue [30]. Diabetes makes changes in angiogenesis, modulates pro-inflammatory cytokine secretion, increases oxidative stress markers, impairs cellular differentiation, and decreases proliferation [31].
With the increasing number of heart attacks and related heart failure, researchers have been searching for stem cells in the heart [32, 33]. VSELs were addressed to help patients after myocardial infarct [33–35], although the low circulating level and lack of significant mobilization triggered by CPB presented in our study was undoubtedly the reason for their wider use.
The surgery itself is a significant injury for the patient. It is known that the greater the interference in our body, the greater the damage and stress and the stronger the catabolic response etc. Looking at all stages of cardiac surgery, we can distinguish several inflammatory factors that may underlie the genesis of mobilization at various stages of the operation. Changing the internal or external environment starts a stress reaction by stressors caused by surgery, called “operational stress” [36]. It occurs due to preoperative factors related to the patient (age, sex), operational (fear, anxiety, drugs, surgeon, extent of tissue damage, hypothermia), and postoperative (pain, infections, hypoxia, immobilization) to modify the neuroendocrine response to trauma [37]. We know that stress can modify the inflammatory response by modulating the immune cell’s economy [38]. The next component of the proinflammatory factor is the surgeon’s activity related with the disruption of tissue continuity. The skin incision and subsequent opening of the chest causes stimulation of the nociceptors located in the skin, subcutaneous tissue, periosteum, and muscle. This is accompanied by the release of inflammation reaction mediators such as cytokines, leukotrienes, TNF, histamines, and prostaglandins. The size of this response is proportional to the extent of the tissue injury [37]. Mobilization triggered by CPB does not exist. The release profile of stem and progenitor cells corresponds with the leucocyte profile released by trauma (psychological and physical), and CPB alone did not intensify this process, even thought the haemodilution effect, which occurs during on-pump operation, was excluded.
The hypothesis made in the study that the extracorporeal circulation as a form of inflammatory reaction is a factor that triggers the mobilization of stem cells from the peripheral blood of the patient was not confirmed in the examined material.