Introduction
Preterm birth, defined as delivery before 37 weeks of gestation, remains a significant public health concern globally, accounting for a substantial proportion of neonatal morbidity and mortality. Preterm infants are particularly vulnerable due to their immature organ systems, predisposing them to various complications, including respiratory distress syndrome (RDS), sepsis, and perinatal asphyxia. In contrast, term infants, born after 37 weeks of gestation, generally have a lower risk of neonatal complications.
Neonatal sepsis is the third leading cause of neonatal mortality, which occurs due to bacterial infection and is a major public health problem, especially in developing countries. Neonatal sepsis is a bloodstream illness that affects newborns under the age of 28 days. It is a significant cause of morbidity and death among neonates, particularly in medium- and low-income countries [1]. Neonatal sepsis is classified into 2 types based on the time of manifestation after birth: early-onset sepsis (EOS) and late-onset sepsis (LOS). EOS refers to sepsis in newborns before 72 hours of life (some experts use 7 days), whereas LOS refers to sepsis occurring after 72 hours of life [2]. The estimated incidence ranges from 5 to 170 per 1,000 births, depending on the socioeconomic setting. The death rate in extremely low birth-weight neonates increases with hospitalisation time, reaching 36% among newborns aged 8–14 days and 52% among infants aged 15–28 days. Its symptoms vary from nonspecific or ambiguous to haemodynamic collapse. Early indications may include irritation, lethargy, or poor eating. Others may have acute respiratory distress, fever, hypothermia, or hypotension, as well as poor perfusion and shock. Sometimes the diagnosis is only assumed based on laboratory results, which may indicate hyperglycaemia or hypoglycaemia, acidosis, or hyperbilirubinemia. Blood culture, the gold standard for diagnosis, is time consuming and insensitive. C-reactive protein and procalcitonin, IL-6, etc., which are now utilised as sepsis biomarkers, are affected by a variety of maternal and foetal pro-inflammatory circumstances throughout the perinatal period [3]. Limitations associated with them include that their levels can get elevated in a variety of inflammatory conditions. Hence, the search is still on for a novel biomarker that holds promise as a tool for evaluating sepsis either as individually or in combination with other biomarkers, to improve the overall sensitivity and specificity for diagnosing and prognosticating bacterial infections.
Cluster of differentiation 14 (CD14) exists in 2 forms, namely membrane-bound (mCD14) and a soluble form (sCD14). The sCD14 has different subtypes that get released in circulation and acted upon by proteases and cathepsin D. The N-terminal fragment of the sCD14-ST subtype is called presepsin. Presepsin (P-SEP) is an immunologic biomarker that offers good accuracy in detecting different infections in adults. Presepsin has aroused interest among researchers, but its utility is still under investigation [4].
Fetuin-A is a heterodimeric plasma glycoprotein containing an A-chain of 282 amino acids and a B-chain of 27 amino acid residues linked by a single inter-disulfide bond. It is predominantly expressed in embryonic cells and adult hepatocytes, and to a lesser extent in adipocytes and monocytes. Fetuin-A binds with a plethora of receptors and exhibits multifaceted physiological and pathological functions. It is involved in the regulation of calcium metabolism, osteogenesis, and the insulin signalling pathway. It also acts as an ectopic calcification inhibitor, protease inhibitor, inflammatory mediator, anti-inflammatory partner, atherogenic factor, and adipogenic factor, among several other moonlighting functions. Fetuin-A has also been demonstrated to play a crucial role in the pathogenesis of several disorders [5–7].
A few studies have also explored the role of presepsin in children [8]. A recent meta-analysis has shown presepsin to have higher sensitivity and diagnostic accuracy, but lower specificity, to detect sepsis in children [9]. Similarly, procalcitonin also has been demonstrated to have usefulness in identifying early-onset sepsis in newborns [10]. Umbilical venous PCT level can also be used to predict adverse neonatal and infantile outcomes related to in utero inflammatory status [11]. In addition, Steinberger et al. observed that the combined determination of PCT and IL-6 from cord blood has more predictive ability for identifying early-onset sepsis in newborns [12].
This study provides an insight into the efficacy of biochemical parameters, i.e. presepsin, procalcitonin, IL-6, and fetuin levels in detecting newborn problems in both preterm and term babies. It also aids in determining which index, among presepsin, procalcitonin, IL-6, and fetuin, is best for predicting newborn problems.
Material and methods
This study employed a prospective observational design to compare the levels of presepsin, procalcitonin, IL-6, fetuin, and CRP in cord blood samples obtained from 176 preterm (88 subjects) and term infants (subjects = 88).
Inclusion criteria
Participants included neonates delivered at a tertiary care hospital.
Preterm infants were defined as those born before 37 weeks of gestation, while term infants were born at or after 37 weeks of gestation.
Exclusion criteria
Exclusion criteria included congenital anomalies, maternal infections (e.g. HIV, hepatitis), and multiple gestations.
Sample collection
Cord blood samples were collected immediately after delivery using standardised procedures. Samples were centrifuged to obtain plasma, which was then stored at –80°C until further analysis.
Biomarker measurement
Levels of presepsin, procalcitonin, IL-6, fetuin, and CRP were measured in cord blood plasma using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions. Maternal and neonatal demographic data, including gestational age, birth weight, APGAR scores, and maternal co-morbidities, were recorded.
Statistical analysis
Descriptive statistics were used to summarise demographic characteristics and biomarker levels. Continuous variables were compared between preterm and term infants using t-tests. The Pearson correlation coefficient test (r) was used to test a positive or negative relationship between 2 (presepsin and fetuin) variables with CRP and procalcitonin. A receiver operating characteristic (ROC) analysis was executed to describe a cutoff value of studied biomarkers for the risk of preterm neonatal sepsis and the associated specificity and sensitivity levels. Results were considered significant if p ≤ 0.05.
Bioethical standards
The study protocol was approved by the institutional Ethics Committee, F. No. SU/2022/1719[2] dated 03/08/2022, and written informed consent was obtained from all participants or their legal guardians.
Results
Neonatal sepsis represents a major cause of morbidity and mortality in neonates. No diagnostic test has been demonstrated to be sufficiently accurate to confirm or exclude neonatal sepsis. This study aimed to evaluate the diagnostic accuracy of various biochemical parameters for neonatal sepsis.
Table I shows a comparison of biochemical parameters between preterm infants and healthy infants. Presepsin values were significantly higher 578.23 ±12.4 in preterm infants compared to healthy infants, i.e. 358.46 ±22.9 (p < 0.001). PCT and IL-6 were also significantly higher than in the healthy infants: 8.62 ±3.81 and 2.2 ±1.03, respectively. Fetuin levels were significantly higher in preterm infants, i.e. 557.32 ±45.62.
Table I
Comparison of biochemical parameters with preterm and term infants
Table II shows the comparison of parameters between those with neonatal complications and without neonatal complications. CRP, presepsin, PCT, IL-6, and fetuin were significantly increased in these variables.
Table II
Comparison of biochemical parameters with neonatal complications and without complications
In Table III, the study shows that infants of mothers with gestational diabetes have higher values of CRP, presepsin, procalcitonin, and fetuin while IL-6 remains the same in both groups, i.e. 33.45 ±3.4, 1,030 ±511, 3.91 ±2.9, 326.9 ±155.4, and 0.97 ±1.8, respectively.
Table III
Comparison of parameters between those with gestational diabetes mellitus (GDM) and those without gestational diabetes
In Table IV the comparison of parameters of infants with/without pre-eclampsia mothers shows high values of CRP, presepsin, and fetuin, while other parameters remain the same, i.e. 41.17 ±17.23, 926.12 ±96.31, and 341.9 ±125.1, respectively, with a p-value of < 0.001 and procalcitonin and IL-6 values 1.91 ±2.9 and 0.92 ±1.8, respectively, with a p-value of < 0.001.
Table IV
Comparison of parameters of infants of pre-eclampsia mothers and infants of non-pre-eclampsia mothers
Table V presents percentage of preterm and term infants with the mode of delivery. High incidences of C-section were seen in the infants (63.63%). Only 39.8% of the infants had vaginal delivery. Preterm and term infants included significantly higher numbers of c-section infants, i.e. 58.03%.
Table V
Percentage of mode of delivery (normal and C-section) with term and preterm infants
| Preterm | Term | |
|---|---|---|
| Mode of delivery (n = 176) | ||
| NVD (39.8%) (n = 64) | 35 (54.6%) | 29 (45.3%) |
| LSCS (63.63%) (n = 112) | 65 (58.03%) | 47 (41.96%) |
Table VI represents the percentages of male and female infants. Number of males is higher in comparison to female infants. Out of the 176 included, only 86 were female infants. A high percentage of preterm infants were male.
Table VI
Percentage of males and females in term and preterm infants
| Preterm | Term | |
|---|---|---|
| Gender (n = 176) | ||
| Male (48.8%) (n = 90) | 59 (65.5%) | 31 (34.4%) |
| Female (51.1%) (n = 86) | 50 (58.31%) | 36 (41.8%) |
Table VII shows a comparison of gestational age distribution of preterm and term infants. The results show a significant difference in preterm and term infants: 36.12 ±12.35 and 38.13 ±23.4 weeks, respectively.
Correlation study
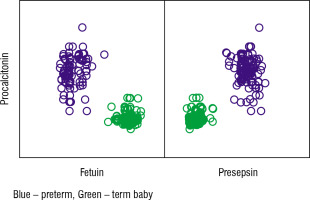
Table VIII shows the correlation between initial levels of CRP and fetuin and presepsin in the preterm and term studied cases. We found a highly significant positive correlation between CRP and presepsin level (p-value < 0.001) and a significant negative correlation between CRP and fetuin (p-value < 0.001; Fig. 1).
Table VIII
Correlation between CRP, fetuin, and presepsin in term and preterm infants
| Fetuin | Presepsin | |
|---|---|---|
| CRP | ||
| Pearson Correlation | –0.847 | 0.858 |
| p-value | < 0.001 | < 0.001 |
| n | 178 | 178 |
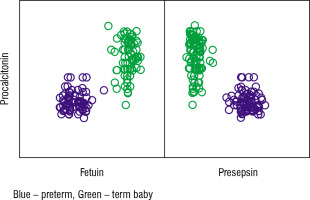
Table IX shows the correlation between procalcitonin, fetuin, and presepsin in the preterm and term studied cases. We found a highly significant positive correlation between procalcitonin and fetuin levels (p-value < 0.001) and a significant negative correlation between procalcitonin and presepsin (p-value < 0.001; Fig. 2).
Diagnostic accuracy of the studied biomarkers for sepsis
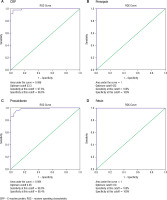
To discriminate between CRP values in the preterm and term group, an ROC curve was created, and we discovered that the AUC of CRP was 0.995 and the optimum cut-off value was set at 33.3, and it had 97.8% sensitivity, and 96.6% specificity (Fig. 3A). Furthermore, regarding the usefulness of presepsin as a marker in the diagnosis of sepsis, ROC analysis showed that the AUC of presepsin was 1.0 (100% sensitivity, 100% specificity), which was higher than the AUC of procalcitonin, at 0.984 (98.9% sensitivity, 85.4% specificity), respectively (Fig. 3B, C). This suggests that presepsin has high specificity and sensitivity for sepsis diagnosis. In addition, fetuin also has an AUC of 1.0 with 100% sensitivity and 100% specificity (Fig. 3D).
Discussion
Despite improvements in neonatal care over the past decades, infections remain common and life-threatening in neonates admitted to the neonatal intensive care unit (NICU). It is important to make an early diagnosis of sepsis because prompt introduction of antimicrobial therapy improves outcomes. However, the diagnosis and management of sepsis are considered a great challenge facing neonatologists, due to nonspecific signs and symptoms, and because laboratory diagnosis is time consuming. Initiation of empirical antibiotic therapy is necessary, and increased multidrug-resistant organisms make the treatment difficult and delay its effect. In the present study, we investigated the levels of biomarkers such as presepsin, procalcitonin, IL-6, and fetuin in cord blood samples of preterm and term infants and their association with sepsis, a kind of neonatal distress. Our findings reveal several important insights into the pathophysiology of neonatal complications and the potential utility of biomarkers in predicting adverse outcomes [13].
Procalcitonin (PCT) is a precursor of calcitonin, and it has been reported to show a probable link with osteogenesis and metabolism of calcium [14] and distress in premature neonates, which are factors that increase the secretion of PCT [15]. In healthy individuals outside the neonatal period, serum PCT concentrations are extremely low (0.01 µg/l). However, after exposure to pro-inflammatory stimuli, especially those of bacterial origin like endotoxins, the concentration rises quickly, within 2 to 4 h, peaks within 6 to 8 h, and remains elevated up to 48 h after stimuli are withdrawn. Numerous authors establish that PCT is a favourable marker for the diagnosis of neonatal sepsis [16, 17]. In these studies, PCT sensitivity in the early diagnosis of NS was found to be 83–100% while the specificity was 70–100%. In agreement of previously reported studies, we also found a significant increase in the level of PCT in preterm neonates in comparison to term neonates (Table I). In addition to this, we also observed a significant increase in various other infants (Tables II–IV). Our study showed that an increased PCT level could be a possible risk factor for severe infection in newborn preterms, while PCT levels were very low in those with no infections.
Interleukin 6 could be used to define the aetiology of sepsis, which is produced by immunoneuclear cells. Its concentration increases quickly after the onset of bacterial infection, with a short half-life. In the past several studies reported that IL-6 may be a noble marker for diagnosis of NS [18]. Its level is increased within a few hours after infection and can be detected in the blood of neonates a few days before the onset of clinical sepsis. A significantly greater inflammatory response in gram-negative sepsis than in gram-positive sepsis has been demonstrated; Celik et al. [19] observed a cut-off level of 202 pg/ml for IL-6, differentiating gram-negative from gram-positive sepsis with 68% sensitivity and 58% specificity. The results obtained from the present study showed that the diagnostic accuracy of IL-6 for predicting NS was not affected by birth-weight of neonates or by any other variables and may be considered as a promising marker for diagnosis of NS.
CRP is the most widely used acute-phase laboratory marker for NS [19]. It plays a critical role in bacterial infection and hence in inflammatory responses. Perrone et al. showed that CRP mean values in healthy children were significantly higher at 48 h of life (4.10 mg/l) than at 24 (2.30 mg/l) and 12 h (0.80 mg/l), and that children born by vaginal delivery and emergency caesarean section had a CRP higher than in those born by elective caesarean section (3.80 mg/l and 3.60 mg/l vs. 2.10 mg/l ). Despite these limitations as a diagnostic marker of sepsis, CRP can be used to exclude sepsis. Normal CRP values in serial controls within a few days from symptom onset are considered indicative of the absence of a bacterial infection. Moreover, CRP can be used to monitor response to antibiotic administration and to decide when antimicrobial treatment can be suspended [20]. CRP in combination with IL-6/procalcitonin improves the diagnostic response.
Presepsin (P-SEP) is the N-terminal fragment of soluble CD14 produced by monocytes and macrophages, in reaction to the pathogen. In the current study, we measured presepsin values in the cord blood of term and preterm infants having risk factors of sepsis, revealing a substantial correlation between cord blood presepsin and onset of sepsis. Previously, various studies supported the use of presepsin levels in cord blood in the diagnosis of sepsis, showing that it can be used as a promising biomarker of inflammation [21, 22]. Although presepsin alone may be sufficient as a predictor for sepsis, in combination with other inflammatory markers, it gives better diagnostic efficacy. Thus, the introduction of presepsin into clinical practice can assist in preventing errors in diagnoses of sepsis, thus preventive neonates’ exposure to antimicrobial drugs and their adverse effects [23]. Finally, P-SEP accuracy was not associated with GA and the method used for marker detection. Moreover, recent studies have led to the definition of P-SEP cut-off values for healthy term and preterm neonates in the first 3 days of life, favouring early identification of neonates.
The mechanisms driving the conditional association of fetuin with foetal growth in GDM are unclear [24]. We speculated that fetuin’s activity might be modified under the GDM-specific intrauterine endocrine conditions. Because there are numerous changes in the fetal endocrine environment in GDM, it is possible that some of these changes may potentiate the effects of fetuin through altering the protein’s post-translational modifications and/or signalling activity. Previous studies have reported variations in fetuin’s phosphorylation or glycosylation levels in growth-restricted and obese individuals. It is plausible that fetuin’s post-translational modifications may be influenced by GDM, which in turn may explain the observed negative association between fetuin and foetal growth in GDM. However, this interpretation is highly speculative because we did not have the data on protein post-translational modifications, which may be the direction for future research [5]. Finally, the correlation of the ROC study was assessed in preterm and term groups, and it was found that these biomarkers might be successfully used as prognostic/diagnostic markers for the early detection of neonatal sepsis with consequent rapid therapeutic decision-making and a possible positive impact on neonatal prognosis (Table VIII–IX and Fig. 3).
The identification of biomarkers associated with neonatal distress has important clinical implications for risk stratification and early intervention strategies. The early detection of infants at risk of neonatal distress using biomarkers could facilitate targeted monitoring and timely intervention, potentially reducing the morbidity and mortality associated with these conditions.
Limitations and future directions
Despite the strengths of our study, several limitations should be acknowledged. The sample size was relatively small, limiting the generalisability of the findings. Future studies with larger sample sizes are warranted to validate our results. Further research is also needed to explore additional biomarkers and their combined utility in predicting neonatal distress, as well as the development of predictive models integrating clinical and biomarker data.
Conclusions
Our study provides valuable insights into the biomarker profiles of preterm and term infants and their association with neonatal distress. The identification of biomarkers associated with adverse neonatal outcomes holds promise for improving risk stratification and clinical management strategies in neonatal care. Continued research in this area is essential to further elucidate the role of biomarkers in neonatal health and disease. In summary, our study contributes to the growing body of evidence on the role of biomarkers in neonatal health and disease and highlights the potential for biomarker-based approaches to improve risk stratification and clinical care in neonatal medicine.

 POLSKI
POLSKI