Introduction
Rare clinical trials were reported on the regimen sofosbuvir (SOF) with daclatasvir (DCV), or simeprevir (SIM) with ribavirin (RBV) for treatment of hepatitis V virus (HCV) relapsers. Current data for patients with treatment failure after a direct-acting antiviral agent (DAA) containing regimen are insufficient. European Association for Study of Liver (EASL) recommendations in 2016 advise retreatment with multiple drugs, sofosbuvir based (because of its higher barrier to resistance), with no cross-resistance with the previously administered drugs, with 1-3 other DAAs plus RBV for 12-24 weeks (24 weeks in patients with F3 and F4). We performed a real world study to determine the efficacy and safety of SOF, DCV, SIM plus RBV in HCV infected patients who failed prior DAA treatment.
Material and methods
Study design
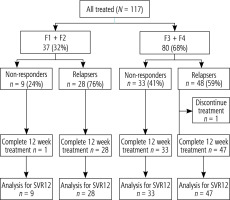
The study design is illustrated in Figure 1. The present cohort includes 117 treatment patients who failed to respond to SOF containing regimens as proved by persistent viremia. Study subjects were randomized according to previous response to therapy to non-responders and relapsers.
The relapses (75 patients) had an end-of-treatment response at the end of their first treatment but relapsed subsequently after the cessation of therapy. The non-responders (42 patients) did not achieve an end-of-treatment response at the end of the first treatment, including patients who had a breakthrough during treatment.
Duration of therapy (12 weeks and 24 weeks) depended on fibrosis stages: SOF, DCV, SIM and weight based RBV for 12 weeks for F1 and F2 and 24 weeks for F3 and F4. Our protocol was performed according to EASL recommendations for treatment of hepatitis C, 2016 [1].
Treatment was with SIM 150 mg once a day, DCV 60 mg once a day, SOF 400 mg once a day, SOF dose was adjusted in patients whose glomerular filtration rate (GFR) was below 30 ml/min. Fibrosis status was assessed using transient elastography.
Written informed consent was obtained from every participant. The institutional review boards approved this study.
Clinical, laboratory and virological follow-ups were done at a monthly interval throughout the treatment period and 3 months after the end of treatment.
HCV genotyping
HCV genotyping was done by direct sequencing of the 5’ untranslated region (5’UTR), using RT-PCR-based assay (AmpliSens HCV-genotype-FRT PCR kit).
Efficacy end points
Treatment efficacy was assessed via measuring HCV RNA viremia at baseline, at 4 weeks, end of therapy and 3 months after completion of the treatment course. HCV RNA was measured using the Roche COBAS Taq Man HCV assay.
Primary virological response was considered achievement of sustained virologic response (SVR) 12, defined as undetectable HCV RNA 12 weeks after completion of therapy.
Secondary virological response included achievement of undetectable HCV RNA after 4 weeks of HCV treatment [rapid virological response (RVR) and end-of-treatment response (EOTR)], defined as undetectable HCV RNA at the end of HCV therapy.
HCV RNA levels were quantified with a lower limit of detection of 15 IU/ml at all sites.
Statistical analysis
Descriptive results are presented as means with standard deviations with interquartile ranges for continuous data. numerical variables were summarized by percentages. Comparisons of baseline variables between patients groups were performed using Fisher’s exact test for categorical variables. P value < 0.05 was considered significant.
Results
One hundred and seventeen patients with chronic HCV who failed to respond to SOF containing regimens as proved by persistent viremia at the end of treatment (non-responders) or those who relapsed subsequently after the cessation of therapy (relapsers) completed the study.
They were recruited and treated using SOF, DCV, SIM and RBV according to EASL 2016 recommendations. Study subjects were randomized according to liver stiffness measurement to two groups: group I, 37 (32%) patients had F1 and F2, and received treatment for 12 weeks, and group II 80 (68%) patients had F3 and F4 and received treatment for 24 weeks.
The mean age range of included patients was 32-65 years, mean 45 years; 78% were male. Patients finished 12, 24 weeks treatment of DCV/SOF/SIM/RBV therapies and 12 weeks of followup after the end of treatment.
The baseline demographic characteristics were reported in two patient groups (Table 1).
Table 1
Baseline characteristics of studied patients
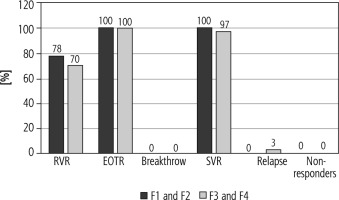
Efficacy in non-responders
Outcomes were available for the 42 patients, undetectable viremia after 4 weeks of treatment initiation (RVR) in previous non-responders patients was 7/9 (78%), 23/33 (70%), respectively, using the per protocol analysis for both groups [group I (F1 and F2) and II (F3 and F4)] (Fig. 2). EOTR and SVR (%) in group I (F1 and F2) and II (F3 and F4) were 9/9 (100%), 33/33 (100%), 9/9 (100%), 32/33 (97 respectively, relapse was 1/33 (3%) in group II (F3 and F4) only. No patients from either group had breakthrough or non-response (Fig. 2).
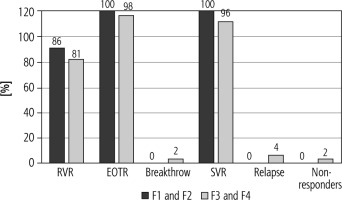
Efficacy in relapsers
Regarding patients with previous relapse, RVR was 23/28 (86%), 38/47 (81%) using per protocol analysis for both groups [group I (F1 and F2) and II (F3 and F4)], EOTR and SVR in group I (F1 and F2) and II (F3 and F4) were 28/28 (100%), 46/47 (98%), 28/28 (100%), 45/47 (96%), respectively. Breakthrough, relapse and non-response were 2%, 4%, 2% respectively only in group II (F3 and F4) (Fig. 3).
Fig. 3
Response rates of patients with previous relapses
RVR – rapid virological response, EOTR – end of therapy response, SVR – sustained virological response Results were calculated based on per protocol analysis
Factors affecting SVR12 rate are compared in Table 2. SVR rate patients was significantly higher in younger age than older only in the non-responders (p < 0.05). The SVR rate in relapsers and non-responders who achieved RVR (undetectable HCV RNA at week 4) was significantly higher than that in patients wo achieved ETR (undetectable HCV RNA at week 12) (81% vs. 16%, p < 0.005 and 71% vs. 26%, p < 0.002, respectively). Regarding modification of RBV dose, the SVR rate in patients with no dose modification was significantly higher than in those with discontinuation of RBV (100% vs. 78%, p < 0.003 and 100% vs. 80%, p < 0.005, respectively). There was no difference in the SVR12 in relation to gender, basal HCV RNA or fibrosis stage in relapsers or non-responders (p < 0.09, p < 0.06 and p < 0.08, p < 0.07 and p < 0.07, p < 0.06, respectively).
Table 2
Comparison of factors influencing the SVR rate in treated patients
Safety and tolerability
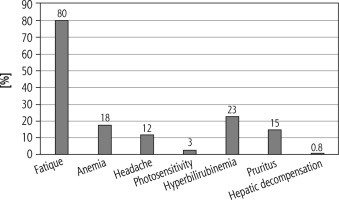
No serious side effects were reported in the studied patients; about 80% of patients reported fatigue, anemia (18%), headache (12%), photosensitivity (3%), hyperbilirubinemia (23%), pruritus (15%) and hepatic decompensation (0.8%) (Fig. 4).
Discussion
The present cohort study aimed to determine the efficacy and safety of SOF with DCV/SMV plus RBV in patients with chronic HCV GT4 who failed to respond to SOF containing regimens as proved by persistent viremia.
Treatment was with SIM 150 mg once daily, DCV 60 mg once a day, SOF 400 mg once a day and weight based RBV for 12 weeks in patients with F1 and F2, and for 24 weeks in patients with F3 and F4.
In patients with HCV genotype 1 or 4 and those with F3 fibrosis or compensated cirrhosis, in whom treatment with an NS5A inhibitor has failed, EASL recommends retreatment with ribavirin plus sofosbuvir, simeprevir, and daclatasvir for 24 weeks [1].
According to European recommendations for the retreatment of HCV patients who failed on a DAA-containing regimen, they should be retreated with a drug with a high barrier to resistance (such as sofosbuvir), plus one or more other drugs, ideally with no cross-resistance with the drugs already administered. Based on results in difficult-to-cure patients, retreatment should be for 12 weeks with ribavirin, or extended to 24 weeks with or without ribavirin [2].
This is the first time that the combination of simeprevir, sofosbuvir and daclatasvir (three drugs with different mechanisms of action) has been assessed in HCV GT4-infected patients with different fibrosis grades.
The SVR rate for previous non-responders was 100% in patients with F1 and F2 and 97% in patients with F3 and F4. In previous relapse the SVR rate was 100% in patients with F1 and F2 and 96% in patients with F3 and F4. Breakthrough, relapse and non-response occurred in previous relapsers only in contrast to the non-responders, indicating that the HCV RNA response to previous treatment is an independent predictive factor for treatment failure.
Lawitz et al. reported that treatment for 12 weeks with simeprevir, daclatasvir and sofosbuvir was generally safe and well tolerated, and the SVR12 was 100%, suggesting that the addition of a third DAA to the simeprevir/sofosbuvir regimen was beneficial in overcoming the limitations of the dual combination when treating patients with cirrhosis [3].
Also, Hézode et al. reported that 60% of DAA experienced patients treated with a combination of SOF/DCV/SMV plus RBV achieved SVR at 12 weeks. The study included a small group of 12 patients only [4].
In contrast to the previously described phase II IMPACT study we did not include patients with decompensated liver diseases or portal hypertension in our study due to limited use of SIM in decompensated patients according to EASL 2016 guidelines.
The combination of SIM and SOF for 12 weeks without ribavirin was assessed in two phase III trials in treatment-naïve and (peginterferon ± ribavirin) treatment-experienced HCV GT1-infected patients with (OPTIMIST-2) [5] or without (OPTIMIST-1) compensated cirrhosis [6]. The regimen demonstrated superiority in SVR12 rates over historical control data in both studies.
OPTIMIST-1, a phase III, randomized study reported that SIM/SOF for 12 weeks has a lower relapse rate than the 8-week treatment [7].
Another phase II open-label study (OSIRIS) was conducted in Egypt. Non-cirrhotic patients were randomized to receive 8 or 12 weeks of treatment whereas compensated cirrhotic patients received a 12-week regimen. SVR12 was 92.1% with all patients treated for 12 weeks regardless of cirrhosis stage [8].
It is important to mention that retreatment with DAA of the same class plus an additional DAA with a different mechanism of action and/or new DAA could achieve high SVR in patients who had previous DAA treatment failure [9].
In our study, patients who achieved RVR had a higher SVR rate than patients who had no RVR in in both relapsing and non-responder groups (81% vs. 16%, p < 0.005 and 71% vs. 26%, p < 0.002) respectively. The SVR rate for young patients was significantly higher than that for older ones in non-responders (100% vs. 93%, p < 0.05). There was no significant difference in other characteristics of the patients in both groups.
In a case report recorded by Safadi et al., retreatment with SOF in combination with SIM resulted in a rapid viral decline at week 2 that resulted in undetectable HCV RNA at retreatment week 4. The patient achieved an SVR at both post-retreatment weeks 12 and 24 [10].
Our analysis showed that the SVR12 rate in patients with no dose modification was significantly higher than in those with discontinuation of RBV in both relapsing and non-responder groups (100% vs. 78%, p < 0.003 and 100% vs. 80%, p < 0.005, respectively).
Consistent with other studies, addition of RBV to the regimen for 12 or 16 weeks increased SVR12 rates to 83% and 89%, respectively, in patients with advanced fibrosis or compensated cirrhosis [11].
Also Buti and Esteban concluded that if re-treatment is needed, the most commonly used strategy is sofosbuvir as backbone therapy plus a drug from a class other than that previously used, for 24 weeks. Moreover, unless it is contraindicated, RBV should also be added to consider triple or quadruple DAA regimens [12].
Only the IMPACT study has evaluated a ribavirin-free version of the same regimen administered for 12 weeks in 40 treatment-naive or treatment-experienced patients. No discontinuation due to adverse events occurred, and a 100% SVR12 rate was achieved. The between-study differences can probably be explained by the fact that our patients had already experience a failed DAA-based regimen.
Concerning safety and tolerability, mild adverse effects were reported and generally were transient. There were no deaths recorded, and only one patient had hepatic decompensation although the patient did not discontinue the treatment.
However, in a study done by Hézode et al., two patients had severe side effects and discontinued treatment; they had advanced liver disease, low platelet counts, and portal hypertension [4]. However, neither had a history of decompensation, and they did not have any indications for liver transplantation.
In contrast, mitochondrial toxicity has not been reported with DAAs, although asymptomatic increases in lipase activity, lactic acidosis and self-limited pancreatitis have been reported with SOF and SIM and with SOF and RBV, indicating that the severe episode of mitochondrial toxicity observed could be treatment related. In addition, although the condition was diagnosed as mitochondrial toxicity at the time, we could not rule out protease inhibitor-induced hepatotoxicity [13].
Conclusions
The current combination regimen was well tolerated and achieved excellent SVR rates with minimal side effects. Therefore, the careful choice of combining multiple DAAs with different viral targets and non-overlapping resistance may be an effective treatment strategy in difficult-to-cure patients, and allow for shorter durations of treatment.