Bioprosthetic valve degeneration poses a significant challenge, particularly in frail, high-risk patients in the clinical setting of hemodynamically unstable decompensated heart failure. The transcatheter approach may be the only therapeutic option in such cases. The intervention on a degenerated bioprosthesis, particularly in the mitral position, bears the additional risk of cerebral embolism, warranting the use of a cerebral protection system (CPS).
We present a case of an 83-year-old male patient with heart failure with reduced ejection fraction, after mitral valve replacement (St Jude Medical 31 mm bioprosthesis implanted in 2012), with a history of infective endocarditis in 2013 and 2021, and multiple comorbidities. The patient was transferred from the orthopedics department, where he was admitted with an intertrochanteric fracture of the left femur, but was eventually found ineligible for surgery due to decompensated heart failure.
On admission, he presented with pulmonary edema, fluid in both pleural cavities requiring thoracocentesis, and massive lower limb swelling. Transthoracic echocardiography (TTE) showed reduced left ventricle ejection fraction (LVEF) (35%), mitral bioprosthesis degeneration with moderate stenosis and severe, eccentric, transvalvular regurgitation (Figure 1 A), and severe tricuspid regurgitation with signs of chronic pulmonary hypertension. Transesophageal echocardiography (TEE) confirmed the dysfunction of the bioprosthesis in the mitral valve with thickened, degenerated leaflets and mobile lesions (Figure 1 B).
Figure 1
A – Transesophageal echocardiography. Degenerated bioprosthetic mitral valve. Bioprosthetic mitral regurgitation. B – Transesophageal echocardiography. Three-dimensional reconstruction of the bioprosthetic mitral valve. C – Fluoroscopy. Aortography and Sentinel cerebral protection system in brachycephalic trunk and left common carotid artery, with both filters in place and fully open (red arrows). D – Fluoroscopy. Optimal position of the implanted valve
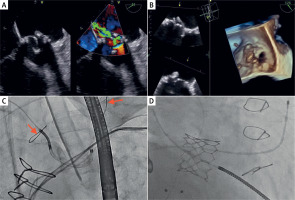
Intensive pharmacological treatment, including high-dose loop diuretics and catecholamines, did not lead to sustainable improvement. After the Heart Team’s decision, the patient underwent percutaneous implantation of the Sapien 29 mm aortic valve in the mitral position (valve-in-valve procedure). The procedure was performed from the right femoral vein access after interatrial septum (IAS) widening with a 12 mm balloon. Perioperative TEE and fluoroscopic assessment confirmed the optimal position of the implanted valve, without residual leak (Figure 1 D). Due to multiple mobile lesions on the degenerative mitral prosthesis, we decided to use the SENTINEL cerebral protection system (Figure 1 C).
In serial follow-up TTE after the procedure, we observed normal function of the implanted valve (Pmean 4 mm Hg, no regurgitation or paravalvular leak). The procedure allowed for hemodynamic stabilization and weaning of the inotropes.
Transcatheter valve-in-valve (ViV) implantation for degenerated mitral bioprosthetic valves is a new therapeutic strategy [1]. It seems to be a feasible, safe, and effective treatment with a procedural success rate of 94.8% [2], and in decompensated, unstable patients may be the only option.
Although cerebral embolic protection systems did not prove beneficial in patients undergoing transcatheter aortic valve implantation (TAVI) [3, 4], it seems justifiable to use them in the scenario of mitral ViV. Even if in this case, the filters of CPS systems were clear after the procedure, the multiple mobile lesions visualized in TEE prompted the use of CPS.
Overall, transcatheter mitral ViV is a viable option in patients with decompensated heart failure due to a degenerated mitral bioprosthesis. Cerebral protection may make the procedure safer when the risk of embolism is high.








