Introduction
Surgical elephant trunk repair (ETR) allows a two-step replacement of the arch and descending aorta in case of diffuse aortic disease [1].
The so-called “frozen” ETR addresses the entire thoracic aorta in a single operation, by means of a hybrid prosthesis, designed to obtain surgical replacement of the aortic arch and contemporary exclusion of the residual disease of the descending thoracic aorta with a self-expanding endovascular prosthesis positioned in an antegrade direction [2]. Although extremely appealing, the frozen ETR requires dedicated material and adequate expertise of the team.
On the other hand, the classic ETR cannot be performed with the newest vascular prostheses, equipped with added branches for the supra-aortic vessels.
Aim
We therefore conceived two different operations that combine the advantages of original ETR coupled with the possibility of individual reimplantation of the supra-aortic vessels, without requiring any specific training or dedicated material other than a regular tri- or tetra-furcated arch prosthesis.
Material and methods
Population and pre-operative assessment
Between January 2018 and December 2019, 8 patients (6 males; mean age: 67.7 ±16 years, range: 50–83 years) were referred to our institution to undergo total arch replacement with a possible, or already advised, need for treatment of the distal aorta. Five, 60 years old or younger, underwent emergency surgery for acute aortic dissection (AAD) with intimal tear in the arch convexity. The remaining 3 suffered from chronic aortic disease involving the arch and the descending thoracic aorta: one expansive aneurysm and 2 chronic dissections. None of the 8 patients needed additional cardiac repairs.
Pre-operative diagnostic tools included transthoracic echocardiograohy (TTE) and computed tomography (CT) scan in all cases. Coronary angiography was performed only in the elective cases. An intra-operative transesophageal echocardiograohy (TEE) was always obtained, as a routine practice in our center.
Surgical technique
Anesthesia management and cerebral perfusion technique are described elsewhere [3]. Briefly, the patient’s monitoring includes cannulation of two peripheral arteries (left and right radial, or left radial and a femoral artery), placement of a cerebral, oxygen-saturation monitoring device (INVOS 4100, Somanetics Corp, Troy, MI, USA), cerebral perfusion pressure line and check of actual cerebral blood flow by means of trans-cranial Doppler.
Proximal repair is usually performed during cooling. Myocardial protection is obtained with selective intra-coronary infusion of antegrade, crystalloid cardioplegia (Custodiol HTK, Essential Pharmaceutics LLC, Durham, NC, USA). HCA is carried out at a core temperature of 25°C.
Cerebral perfusion is obtained with a standard Kazui technique when a common femoral artery is used as the site for arterial return. If the selected site is the right axillary artery, the systemic perfusion is lowered to 0.5 l/min immediately before brachio-cephalic trunk clamping, temporary unilateral brain perfusion through the right axillary-carotid system and hypothermic circulatory arrest (HCA) are established, and the arch opened. The left common carotid artery is then cannulated through its lumen, bilateral brain perfusion is started, and the left subclavian artery is clamped. Cerebral flow is then adjusted based on the INVOS signal, infusion pressure and intracranial blood flow measurement, and so the arch is resected completely, preserving the origins of the supra-aortic vessels.
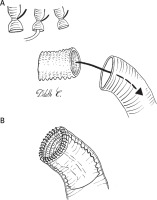
Type 1: modified ET
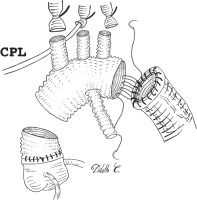
A 6–8 cm segment of vascular prosthesis is prepared, either cutting the distal end of a tetra-furcated prosthesis (Intergard Woven Aortic Arch, Getinge AB, Göteborg, Sweden), or using a different, straight vascular graft of appropriate diameter (Figure 1 A). This independent segment will become the “elephant trunk” and serve as a landing zone for a subsequent thoracic endovascular aortic repair (TEVAR). It is inserted in the descending aorta, and its proximal end is anastomosed to the distal aortic stump with a 4.0 polypropylene continuous suture, reinforced with a peri-adventitial strip of Teflon felt (Figure 1 B). The arch prosthesis is then anastomosed to the aortic stump, fitted with the ET graft, by means of a 3.0 polypropylene continuous suture that is passed through the ET graft itself, the aortic wall, the Teflon felt strip and the arch graft (Figure 2). The distal collateral branch of the arch prosthesis is anastomosed end-to-end to the left subclavian artery origin with a 5.0 polypropylene continuous suture, reinforced with a peri-adventitial strip of Teflon felt. The aortic arch prosthesis is clamped between the distal and central branch, the distal aorta is de-aired, and the service branch cannulated: lower body perfusion is restarted, while cerebral perfusion is continued on an independent rotor. Once the anastomosis between the central branch and left carotid artery is completed, the aortic clamp is repositioned between the proximal and central branch, and the intra-luminal cannula is removed. Right-sided cerebral perfusion is obtained through the right axillary artery or the intra-luminal cannula in the brachio-cephalic artery, whilst left-side flow is allowed through the service branch and the arch. The last anastomosis of the supra-aortic vessels between the proximal branch and anonymous artery is performed in the same way. Once completed, the aortic clamp is repositioned proximally to the first branch origin. Whole body perfusion is therefore achieved through the arch lumen, and proximal repair can be completed as needed.
Type 2: “prophylactic debranching”
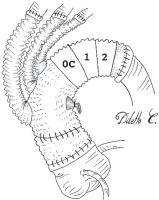
The distal tail of the arch prosthesis is simply left uncut, or barely shortened. This branch-free segment will actually replace the arch (zone 0C-2). Therefore, the branched portion of the graft will be dislocated proximally (zone 0A-B). Distal anastomosis will be performed at the distal aortic stump site with a 3.0 polypropylene continuous suture, reinforced with both an endo-luminal and peri-adventitial Teflon felt strip. Subsequent surgical steps are superimposable to those described for type 1 repair. The final aspect will be similar to a conventional arch debranching, with the three supra-aortic vessels originating from the ascending tract. The prosthetic arch, freed from all collaterals, will serve as a landing zone for subsequent TEVAR (Figure 3). Proximal repair is then completed as requested.
Results
A single patient died, due to multi-organ failure and sepsis. Re-exploration for bleeding was necessary in one further patient. Three patients suffered transient cerebro-vascular accidents. One needed prolonged antibiotic therapy for suspected prosthetic infection. Two patients underwent prolonged mechanical ventilation for temporary respiratory failure. No strokes or spinal cord injuries were detected.
Discussion
Aortic arch replacement remains a challenging operation. When the descending thoracic aorta is involved, treatment should be extended distally, increasing operation complexity and surgical risk. Distal completion can be obtained after a classic ETR, thanks to the prosthetic segment left in the descending aorta, or through a frozen ETR procedure, which encompasses contemporary arch replacement and endo-luminal exclusion of distal aortic disease.
The first is an established operation, providing excellent results [4]. Nonetheless, the distal anastomosis with the invaginated prosthesis is demanding, especially in AAD. Moreover, it requires the use of a straight vascular prosthesis and the reimplantation of an aortic cuff including the origins of the supra-aortic vessels: a long anastomosis that can be very difficult to re-explore once completed.
On the other hand, frozen ETR requires the availability of dedicated material and adequate whole-team expertise, the latter not being easy to acquire, due to the rarity of the disease.
We therefore conceived two possible alternatives to the classic procedure that are easier to perform, while maintaining the possibility of a safe secondary correction of residual disease in the descending aorta.
The first operation is a true “modified ETR”. A similar, although much more complex, modification has already been proposed in the past [5]. In our technique, the ET is independently anastomosed inside the distal lumen. Therefore, the suture is easier to perform, due to increased visibility and simpler graft handling. The time needed for the additional suture line between the arch prosthesis and the distal aortic stump equipped with the “trunk” should be compensated by these technical advantages. Also the three supra-aortic sutures will be completed more quickly than the cuff anastomosis, and a shorter HCA time will be obtained if an additional “service branch” for distal perfusion is available.
Another advantage is that the distal skirt diameter can be freely chosen to accommodate for potential dimensional discrepancies.
The second operation might be considered a “prophylactic arch debranching”. The technique is just based on leaving a distal tail in the prosthesis, after the origin of the side branch for left subclavian artery. The branch-free tail will actually substitute the transverse arch. The origins of the supra-aortic vessels will be therefore displaced proximally, and they will assume the position that they would have taken after a typical surgical arch debranching. This avoids the need for a second open procedure by preparing a zone 1 landing during the first procedure.
“Modified ETR” should be more useful in the case of chronic expansive disease, where the positioning of the free-flowing prosthetic segment is easier to accomplish [6]. “Prophylactic debranching” should be more convenient in AAD, since it requires a single distal suture line and forestalls the insertion of any prosthetic segment in a fragile, and scarcely visible, distal lumen.
Both techniques appear to be reproducible and easy to perform, avoid difficult maneuvers included in the original ETR, do not require any particular expertise or specific training, and do not need the use of dedicated material.