Introduction
Thoracic esophageal diverticulum (TED) is a rare benign esophageal disorder which can present with dysphagia, vomiting or chest pain. Its etiology remains unclear, but it is associated with esophageal motility disorders [1, 2].
TED can also be described as a pulsion diverticulum, a type of pseudodiverticulum which develops secondary to a motility disorder. These pseudodiverticula develop just above an area of increased pressure or uncoordinated peristalsis, which causes an increase in pressure inside the esophagus [1, 2]. In terms of location, TED may be epiphrenic, or form in the mid-esophagus, 5 cm above or below the carina [2, 3]. The former are mainly, in about 75% of cases, secondary to motility disorders, and the latter, which are true diverticula (Rokitansky diverticula), may result from traction of the esophageal wall by post-inflammatory scarring from mediastinal inflammation, e.g. tuberculosis [4, 5]. In the middle part of the esophagus, pulsion diverticula may occur and the treatment methods are similar to that of epiphrenic diverticula. The third group of esophageal diverticula comprises iatrogenic diverticula, which occur as a result of an endoscopic diverticular peroral endoscopic myotomy (D-POEM) procedure [6, 7].
Aim
Surgical treatment of the diverticula of the thoracic esophagus is a difficult clinical problem. We present our analysis of patients treated over a 20-year period.
Material and methods
Patients
The study is retrospective, based on hospital records; no consent was obtained from the Ethics Committee.
A retrospective analysis of 26 patients who underwent operative management of the diverticula of the thoracic esophagus during the period 1998 to 2018 was conducted. Inclusion criteria were patients undergoing surgical treatment for symptomatic thoracic esophageal diverticula who were operated on in the Department of Thoracic Surgery, John Paul II Hospital, Jagiellonian University Collegium Medicum. Routine diagnostic work up included contrast examination (a barium esophagram), chest computed tomography (CT scan), spirometry and, selectively, manometry. The degree of dysphagia and general condition were evaluated. Patients underwent transthoracic resection of the diverticulum, myotomy and some of them anti-reflux surgery. Perioperative complications were assessed using the Clavien-Dindo scale [8].
The degree of dysphagia was assessed using a 4-point scale [9]: 0 – no dysphagia; 1 – able to swallow a semi-liquid diet; 2 – able to swallow a liquid diet; 3 – dysphagia to liquids and saliva.
Methods
A thoracic or abdominal approach was used in all patients undergoing surgery. In the thoracic approach, the incision was made either on the right or the left side depending on the location of the diverticulum. During surgery the diverticulum was identified. A 45 Fr calibrated bougie or gastroscope was inserted into the esophagus and the diverticulum was resected with a 1cm margin in order to prevent stricture formation. The diverticulum was either resected using a stapler device (TA-30 Ethicon, USA, Endo-GIA 60 Auto-Suture, USA), or excised and sutured manually. The stapler suture line was sutured using running 000 Vicryl. Next the esophagus was rotated 180° and esophagomyotomy of 5–12 cm was performed on the esophagogastric junction, and onto the stomach for 1–2 cm. Integrity of the suture line was confirmed by insufflation of air using the gastroscope, with the esophagus immersed underwater. Additional buttressing of the suture line was not performed. Among the patients who were scheduled for anti-reflux surgery, left-sided thoracotomy access was the preferred option. Manometry was routinely performed from 2012.
Postoperative period
Nutrition
On the first day after surgery, the patients were fed via a feeding tube, and in the case of enteral intolerance, parenteral nutrition was commenced. From the 3rd postoperative day, patients were fed a liquid diet. Routine contrast examination, with optional esophagoscopy, was performed on day 7 following surgery. For patients with achalasia who did not undergo resection of the diverticulum, a liquid diet was introduced on the 1st postoperative day. All patients were advised to maintain a small particle diet for a period of 14 days after discharge from hospital.
Follow-up
A routine chest X-ray was performed on the day following surgery. The patients’ quality of life was assessed 30 days after surgery, then every 3 months in the first year and once a year in subsequent years. If a follow-up check on site was not feasible, patients were interviewed by phone. During each follow-up visit, patients underwent contrast examination and/or CT scan, and dysphagia and general condition and comfort were assessed using the Visick scale [10].
Statistical analysis
All statistical analyses were performed with the Statistica 10PL software package (StatSoft, USA). For demographic and clinical data descriptive statistics (mean value, median and range) were used. The non-parametric Wilcoxon-Gehan test was used to assess the degree of dysphagia before and after surgery. Qualitative variables were expressed as a number and percentage and were compared using the χ2 test and Fisher’s test. P < 0.05 was considered statistically significant.
Results
Patients
A total of 26 patients underwent surgical treatment for esophageal diverticulum, of which 19 (73.1%) were male and 7 (26.9%) were female. The median age was 64.2 years (range: 45–86 years). Twenty-four (92.4%) patients underwent elective surgery, with 2 (7.7%) being treated following acute presentation. In 23(88.5%) a diverticulum resection was performed and 3 (7.7%) withdrew from the resection due to the small size of the diverticulum and patients were followed up. Manometry was performed in 8 (30.8%) patients. In 3 (11.5%) esophageal manometry was normal, and in 5 (19.2%) high amplitude (160–180 mm Hg) with progressive peristalsis was found.
Symptoms
All patients presented with symptomatic esophageal diverticula. In the group of treated patients, the dominant symptoms were dysphagia, chest pain regurgitation, odynophagia and halitosis which had been present for a duration of 12–56 months (mean: 22.6 months) (Table I). Relief was observed in all of the patients who presented with dysphagia. In the preoperative evaluation, the degree of dysphagia in patients ranged from 2 to 3 with a mean of 2.19, and after surgical treatment 1–2 with a mean of 1.2 (p = 0.0023). Weight loss was present in 15 (57.7%) patients, ranging from 4 to 12 kg (mean: 5.8 kg). All patients with weight loss returned to pre-disease weight after surgical treatment (p = 0.0035).
Table I
Patient characteristics
Thoracic diverticula
In 19 (73.1%) patients the diverticulum was located in the epiphrenic region, and in the remaining 4 (15.4%) patients the diverticulum was located in the mid-esophagus. The main symptoms were progressive dysphagia, chest pain and regurgitation.
Epiphrenic diverticulum
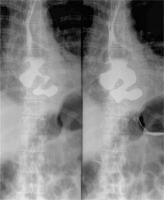
Among 19 (73.1%) operated patients the size of the diverticula ranged between 4 and 8 cm (mean: 5.7 cm) (Figure 1).
Diverticulum with achalasia – In the 5 (19.2%) patients with concomitant achalasia, the diverticulum size ranged between 2 and 6 cm (mean: 3.2 cm). In 2 (7.7%) patients the diverticulum size was 3 and 5 cm and in 3 (11.5%) patients the diverticula measured 2 cm each.
Patients with acutely symptoms – Two (7.7%) patients presented acutely with symptoms of mediastinitis requiring emergency treatment. In the first such patient the diverticulum had perforated, resulting in a right lower lobe abscess, and the second patient was operated on laparoscopically in a different department and had a suspected fistula diagnosed on the first postoperative day.
Surgical treatment
A right thoracotomy approach was used in 14 (53.8%) patients, a left thoracotomy approach in 9 (34.7%) and laparotomy in 3 (11.5%) patients. In 9 (34.7%) patients, a long myotomy was performed, in 14 (53.8%) local myotomy (5 cm long), and in 3 (11.5%) the Heller-Dor procedure. Nine (34.7%) patients underwent anti-reflux surgery, of whom 5 (19.2%) underwent Belsey Mark IV fundoplication and 4 (15.4%) underwent Dor fundoplication. In patients with achalasia, 5 (19.2%) underwent myotomy with anti-reflux surgery. In 2 (7.7%) a left thoracic approach with long myotomy was performed and in 3 (11.5%) an abdominal approach with the Heller-Dor procedure. In these 3 (11.5%) patients with diverticula measuring 2 cm, the diverticulum was left unresected after intraoperative endoscopic evaluation, as the small size and wide neck of the diverticula would not be an obstacle to the passage of food. In 1 (3.8%) patient the surgery was extended with a wedge resection of the right lower lobe. In 18 (69.2%) patients the diverticulum was excised using a stapling device and in 5 (19.2%) patients it was resected using manual suturing with Vicryl 000.
The median length of stay after surgery was 10 days (range: 7–39 days).
Perioperative complications according to Clavien-Dindo classification [8] (Table II)
Table II
Surgical complications according to the Clavien and Dindo classification
Major perioperative complications
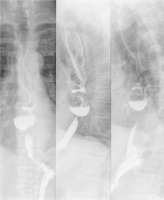
In 2 (7.7%) patients an esophageal leak was diagnosed with septic complications (IV). Both patients suffered respiratory failure requiring mechanical ventilation. The first patient required ventilation for 21 days and the fistula spontaneously closed following conservative treatment. In long-term follow-up and endoscopic examination of this patient, there was no stricture of the esophagus and the patient was on a full diet. In the second patient double diverticula resection was performed (Figure 2) and unfortunately there was a fistula, re-closure was also ineffective, the patient underwent esophagectomy and esophagostomy and was scheduled for reconstruction with colonic interposition (IIIb). In the postoperative course the patient required mechanical ventilation for 5 days (IV). In the remaining patients the postoperative period was uneventful. There was no mortality in the perioperative period of hospital stay.
Minor postoperative complications
Postoperative wound infection occurred in 2 (7.7%) patients (I). Three (11.5%) patients required bronchoscopy and aspiration secretions from the bronchial tree during the postoperative period (III). One (3.8%) patient experienced symptoms of postoperative psychosis (I) and 1 (3.8%) patient suffered a postoperative urinary tract infection (I).
Late complications
Recurrence of diverticulum was not observed in this study. In 1 (3.8) patient following colonic reconstruction, an anastomotic stricture was diagnosed and this patient required dilatation once every 12 months. One (3.8%) patient reported recurrence of symptoms, not confirmed on diagnostic testing with CT scan and esophagoscopy and required conservative management only.
General comfort of patients after surgery
In the long-term follow-up, 19/23 (73.1%) patients were evaluated using the Visick scale [12]. A very good result was achieved for 7 (26.9%) patients, a good result for 11 (42.3%) patients, and a bad result for 1 (3.8%) patient.
Discussion
TED is a rare benign esophageal disorder. The prevalence of esophageal diverticulum is estimated at 0.0015% in the USA, 0.77% in Japan and approximately 2% in Europe [11].
The criteria for surgical treatment are not entirely clear. It is believed that a diverticular size of over 5 cm that results in clinical symptoms is an indication for surgical treatment. Altorki et al. believe that regardless of symptoms, epiphrenic diverticula should be resected [12]. In the presented paper only patients with clinical symptoms were qualified for surgical treatment.
Among patients treated for esophageal epiphrenic diverticula, motility disorders are found in 60–100% of patients [13–15]. Therefore, it is important to identify patients with elevated intraluminal pressure, which is responsible for the formation of epiphrenic diverticula. For these patients, resection of the esophageal diverticulum is insufficient and esophageal myotomy is required.
There are three surgical treatment options: conventional open thoracic or abdominal approach; minimally invasive approach via either laparoscopy, thoracoscopy or robotic technique; and from 2010, the endoscopic approach. Because of their decreased invasiveness, the minimally invasive techniques are the preferred approaches, but an open thoracic approach remains one of the most commonly used techniques [16–21].
Performing myotomy of diverticula is still a subject of debate. Myotomy is necessary to decrease intraesophageally pressure and therefore lower the risk of esophageal leaks.
Varghese et al. and Belsey propose a long myotomy from the aortic arch to the lower esophageal sphincter (LES), but Streitz et al. suggest performing myotomy only on the segment of the esophagus with abnormal manometry, while others suggest extending myotomy from the diverticulum neck to the proximal 1.5 cm and 2 cm of the stomach [3, 13, 22]. Westcott et al. propose only a myotomy and leaving the diverticulum, and resection when symptoms recur [23]. In our study short myotomy was performed in 14 (53.8%) patients. Esophageal leak occurred only in this group. In 1 patient, the cause could be related to a double diverticulum, the resection line was long, approx. 6 cm, which could lead to wall ischemia and fistula, and in the second patient, the diverticulum and esophageal wall were altered by an inflammatory reaction drawing of the esophagus wall, which resulted in a too deep diverticulectomy and the occurrence of the fistula. We associate the occurrence of the above-mentioned complications with technical difficulties in diverticulum resection rather than with the type of myotomy – in the first case, with a long resection line of two diverticula, and in the second case, with an inflammatory process that makes it difficult to close the defect of the esophageal wall. In such difficult cases, a long myotomy is justified taking into account the results of the manometric examination.
Tapias et al. propose that the suture line should be buttressed with a viable tissue, e.g. intercostal muscle, and emphasize the importance of meticulously performed diverticulectomy and myotomy surgery. In their analysis the esophageal leak incidence rate was 3.2% [11]. This is the most commonly reported postoperative complication which is reported to occur in up to 10–20% of cases [14, 20, 21]. In many studies, including ours, there were no fatal postoperative complications, but in previous studies the mortality rate ranged from 0 to 10% [20, 21]. Using a laparoscopic approach, the percentage of esophageal leaks is similar [6, 17, 18].
Diverticulum resection is a very important element of surgical treatment. In our procedure, we suggest leaving a 1 cm margin to avoid postoperative esophageal stenosis. In the resection technique, we use a Bougie 45 Fr or a gastroscope, simultaneously assessing the tightness of the suture line. Leaving a larger margin and inaccurately performed myotomy may result in a recurrence of the diverticulum or esophageal fistula.
Long myotomy is mandatory for patients with an epiphrenic diverticulum and diffuse esophageal spasm. In patients with a hypertensive and unrelaxing LES, myotomy from the upper margin of the diverticulum to 1–2 cm below the cardia should be performed [3, 14]. Cardiomyotomy is not recommended in patients with a normotensive or hypotensive LES [3]. In patients undergoing cardiomyotomy, partial fundoplication should also be performed. This type of myotomy and anti-reflux surgery was performed in 9 (34.6%) patients in whom the diverticulum was located in the lower part of the esophagus. The preferred type of anti-reflux surgery was the incomplete procedures of Belsey, Toupet or Dor. Tapias et al. performed anti-reflux surgery in 12.9% of their population and emphasize the lack of improvement in clinical symptoms after the procedure [11]. Authors using a laparoscopic approach are of the opposite opinion and prefer to perform anti-reflux surgery in their patients [16, 17]. In our study, 3 patients underwent myotomy with anti-reflux surgery, and the diverticula, measuring 2–3 cm in diameter, were left unresected. Herbella and Patti and Westcott et al. propose that small diverticula may be left and not resected and this did not result in persistent dysphagia symptoms, with patients experiencing clinical improvement after surgery [18, 23].
In our study, patients were followed up long term for a mean of 43 months. During follow-up, 7 (30.4%) patients achieved a very good result, 11 (47.8%) had a good result and 1 (4.3%) had a bad result, and required esophageal dilatation once a year. Other authors also report very good results in long-term follow-up [11, 12, 19, 20].
Conclusions
Surgical treatment of TED using an open approach is a safe procedure, but with a relatively high rate of perioperative complications. The complication rate is comparable between laparoscopic and open approaches. Resection of an esophageal diverticulum with myotomy should be the standard treatment, with anti-reflux surgery recommended in cases of epiphrenic diverticulum, concomitant diffuse esophageal spasm and in diverticula with achalasia. Manometry, particularly high-resolution manometry, should form part of the routine diagnostic workup [24].