Hemoptysis, defined as the expectoration of blood originating from the lower respiratory tract, represents a potentially life-threatening medical condition requiring prompt diagnosis and treatment. While minor hemoptysis is often self-limiting, massive hemoptysis, characterized by blood loss exceeding 300–600 ml in 24 hours, carries a high risk of morbidity and mortality primarily due to asphyxiation rather than exsanguination [1, 2]. Massive hemoptysis accounts for less than 5% of all cases but necessitates immediate and effective intervention to prevent fatal outcomes [3, 4]. The underlying etiology of hemoptysis varies geographically and includes pulmonary infections, malignancies, bronchiectasis, and vascular anomalies such as arteriovenous malformations. In regions with a high prevalence of tuberculosis, infection-related hemoptysis is most common, whereas malignancies dominate in developed countries [4, 5]. Non-bronchial systemic arteries, including the phrenic and intercostal arteries, are occasionally implicated, contributing to diagnostic and therapeutic challenges [6]. Bronchial artery embolization (BAE), first described by Remy et al. in 1973, has emerged as the first-line treatment for massive hemoptysis. This minimally invasive procedure effectively controls bleeding by occluding hypertrophic and abnormal bronchial arteries, thus reducing the need for high-risk surgical interventions [3, 7]. Advancements in imaging modalities, such as multidetector computed tomography (MDCT) and angiography, have further enhanced the precision of BAE, enabling detailed visualization of vascular anatomy and aiding in the localization of bleeding sources [8, 9]. Despite its effectiveness, BAE is associated with recurrence rates ranging from 10% to 57%, often due to incomplete embolization, recruitment of collateral vessels, or progression of underlying disease. Moreover, complications such as spinal cord ischemia, although rare, underscore the importance of meticulous technique and the use of superselective embolization strategies [4, 6]. This case report illustrates the role of BAE in managing massive hemoptysis secondary to bronchiectasis, highlighting diagnostic and therapeutic considerations.
A 32-year-old male patient, an active smoker with a 15-year history of more than 45 pack-years, presented to the emergency department with a 12-hour history of dyspnea and severe coughing accompanied by bloody sputum. The symptoms, notably worsened during nighttime, caused significant discomfort. The patient had a medical history of untreated hypertension but no prior use of antiplatelet or anticoagulant medications. He denied any recent fever, chest pain, trauma, or surgical procedures. On examination, he was hemodynamically stable, with a blood pressure of 180/110 mm Hg, a pulse rate of 95 beats per minute, a temperature of 36°C, and SpO2 of 97%. He expectorated approximately 10 ml of bright red blood. Respiratory examination revealed bilateral diminished breath sounds without additional findings. Arterial blood gas analysis was within normal limits (pH 7.43, PaO2 96 mmHg, PaCO2 35 mm Hg, HCO3 – 24 mmol/l). Initial management included intravenous fluids, tranexamic acid, antibiotics (ampicillin/clavulanic acid and azithromycin), and cough suppressants (codeine). Diagnostic tests for bronchitis, tuberculosis, collagen vascular diseases, and endobronchial lesions were negative. Computed tomography revealed bullous lung disease, bilateral bronchiectasis, and ground-glass opacities predominantly affecting the upper and middle lung fields (Figure 1). Bronchoscopy identified an active bleeding site in the right upper lobe (segment B1). Blood clots in the right middle and lower lobes were removed, while the left lung appeared clear. Despite initial hemostasis with tranexamic acid, the patient experienced a recurrence of hemoptysis 3 days later, expectorating approximately 200 ml of bright red blood. The patient’s respiratory condition deteriorated, necessitating intubation and preparations for a right upper lobectomy. Prior to intervention, a second bronchoscopy was performed, revealing an additional bleeding site in the right lower lobe (segment B10). Local hemostasis was achieved using endobronchial instillation of adrenaline and tranexamic acid. The patient was subsequently admitted to the intensive care unit for 4 days. Further investigation with bronchial artery angiography (BAA) demonstrated a pathological branch of the right bronchial artery supplying both the right upper and lower lobes, identified as the source of bleeding. This branch was successfully embolized using 300–500 μm microspheres (Embosphere – Merit Medical). Hemostasis was immediately achieved, and the patient showed no recurrence of hemoptysis (Figure 2). The patient remained hemodynamically stable and was discharged from the pulmonology clinic 7 days later in good condition, afebrile, and free of respiratory symptoms. Follow-up evaluations at 1 month, 6 months, 1 year, and 5 years after the procedure demonstrated no recurrence of hemoptysis, confirming the long-term success of the intervention.
Figure 1
Computed tomography (CT) image demonstrating emphysematous lung parenchyma (blue arrow) and the bleeding site in the bronchial artery (green arrow), identified as the source of hemoptysis
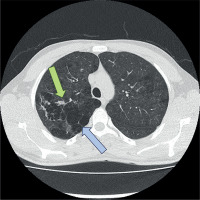
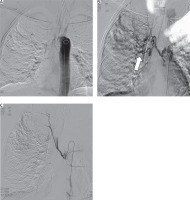
Figure 2
Interventional angiographic identification and embolization of the bronchial artery. A – Introduction and advancement of the catheter into the descending aorta at the level of the bleeding bronchial artery. B – Angiographic identification of the bleeding artery (arrow) with active extravasation of contrast indicating hemorrhage. C – Post-embolization angiographic image showing successful occlusion of the bronchial artery with cessation of bleeding
Massive hemoptysis is a critical condition with a high mortality rate if untreated, necessitating a multidisciplinary approach to management. The primary goals include airway protection, identification of the bleeding source, and cessation of hemorrhage [1, 2]. While bronchoscopy and chest radiography are essential initial diagnostic tools, MDCT with angiographic capabilities has become the gold standard for identifying the anatomical source of bleeding, especially in cases involving non-bronchial systemic arteries [6, 8]. BAE is recognized as the most effective non-surgical intervention for massive hemoptysis. Its technical success rates exceed 97%, with immediate clinical success observed in over 90% of cases [3, 4]. In this case, selective embolization using advanced catheterization techniques and embolic agents effectively controlled the hemorrhage. Polyvinyl alcohol particles and coils are commonly used embolic materials, but N-butyl-2-cyanoacrylate (NBCA) has demonstrated superior efficacy in reducing recurrence rates [3, 4]. In rare instances, hemoptysis arises from non-bronchial systemic arteries or vascular malformations, as seen in this case involving a phrenic artery-to-pulmonary artery fistula. Such anomalies emphasize the importance of comprehensive vascular imaging and tailored embolization strategies to achieve hemostasis [6, 9]. Recurrent hemoptysis following BAE remains a significant challenge, with risk factors including incomplete embolization, recanalization of previously treated vessels, and the recruitment of collateral circulation [5, 6]. This underlines the importance of addressing underlying conditions, such as bronchiectasis or infection, through concurrent medical management [2, 10, 11]. Additionally, repeat embolization is often necessary in cases of recurrent bleeding, as highlighted in studies demonstrating successful outcomes with subsequent interventions [3, 7, 12]. Dong et al. [12] identified underlying vascular anomalies and the use of suboptimal embolic materials as key predictors of recurrence, emphasizing the need for thorough pre-procedural planning. Similarly, Garcia-Olivé et al. [13] demonstrated that angiographic findings, such as large-caliber arteries and systemic-pulmonary shunting, significantly increase the likelihood of recanalization. In a retrospective analysis, Dabó et al. [14] demonstrated the importance of addressing underlying pulmonary conditions, such as bronchiectasis or tuberculosis, to enhance long-term outcomes. Furthermore, Abid et al. [11] highlighted the effectiveness of superselective embolization techniques in reducing recurrence rates while maintaining a favorable safety profile. These findings collectively underline the necessity of a multidisciplinary approach that combines advanced imaging, precise embolization techniques, and targeted management of underlying diseases to improve procedural success and reduce recurrence. The safety profile of BAE is well documented, with major complication rates below 1%. However, spinal cord ischemia remains the most feared complication due to inadvertent embolization of spinal artery branches. Superselective catheterization and meticulous angiographic evaluation are critical to minimizing this risk [4, 7]. Overall, BAE nowadays constitutes a cornerstone in the management of massive hemoptysis, offering high success rates and a favorable safety profile. The integration of advanced imaging techniques and embolization materials continues to enhance its efficacy, particularly in complex cases involving atypical vascular sources. This case underlines the importance of a personalized approach to the management of hemoptysis, combining prompt intervention with targeted treatment of underlying pathologies. Bronchial artery embolization is an effective and minimally invasive intervention for managing massive hemoptysis, offering high rates of immediate hemostasis and a favorable safety profile. This case highlights the importance of advanced imaging modalities for precise localization of bleeding sources, as well as the role of super selective embolization techniques in achieving durable outcomes. Despite its efficacy, the risk of recurrence underscores the need for a comprehensive approach that addresses underlying pathologies and incorporates repeat interventions when necessary. As advancements in embolization materials and techniques continue, BAE remains the cornerstone of treatment for massive hemoptysis, offering life-saving benefits and improved quality of life for affected patients.






