Introduction
Heart injury and hemodynamic abnormalities are common complications in cirrhotic patients [1-5]. Cardiac contraction abnormalities and decreased cardiac output, besides arrhythmia and heart failure, could occur in cirrhosis [1-5]. Liver transplantation is the only accepted standard intervention for the management of cirrhosis-associated cardiac impairment. Unfortunately, there is a scarcity of cellular and molecular studies on the mechanisms involved in cardiac impairment induced by cirrhosis. On the other hand, no particular pharmacological intervention has been developed to prevent heart tissue injury in cirrhotic patients.
Taurine (Tau) is the most abundant non-protein amino acid in the human body. Several physiological as well as pharmacological properties have been attributed to Tau [6-8]. It has been found that Tau treatment effectively blunts xenobiotic-induced organ injury [9-15]. Moreover, it has been well documented that several diseases could benefit from exogenous Tau supplementation [6, 16-19].
The beneficial effects of Tau have been demonstrated in a wide range of cardiovascular diseases [20-22]. The positive effects of Tau on high plasma cholesterol, coronary artery diseases, congestive heart failure, and myocardial function have been documented [20-22]. The antioxidant role of Tau has been mentioned as a fundamental mechanism involved in the cardioprotective effects of this amino acid [23-25]. It has been found that Tau protects heart tissue against toxic insults by alleviation of oxidative stress and its associated events [10, 26-31]. Interestingly, recent studies mentioned that the antioxidant effect of Tau is indirectly mediated by the effects of this amino acid on cellular mitochondria [16, 32-37].
It has been found that Tau significantly improved mitochondrial function and energy metabolism [16, 38, 39]. Tau could also significantly decrease mitochondria-derived reactive oxygen species (ROS) [40-43]. The release of cell death mediators from mitochondria and suppression of mitochondria-mediated cell death are also evident upon Tau administration [40, 44, 45].
The current study was designed to evaluate the role of mitochondrial dysfunction and oxidative stress in the heart tissue of cirrhotic animals. Additionally, the impact of Tau supplementation on cardiac dysfunction in cirrhosis was monitored.
Material and methods
Chemicals
2,4,6-tripyridyl-s-triazine (TPTZ), 3-[4,5dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), 3-(N-morpholino) propane sulfonic acid (MOPS), Coomassie brilliant blue, 2’,7’-dichlorofluorescein (DCFH), 6-hydroxy-2,5,7,8-tetramethylchroman-carboxylic acid (Trolox), dithiothreitol (DTT), fatty acid-free bovine serum albumin (BSA) fraction V, ferric chloride hexahydrate (FeCl3•6H<sub>2</sub>O), D-mannitol, reduced glutathione (GSH), malondialdehyde (MDA), sodium succinate, rhodamine 123 (Rh 123), oxidized glutathione (GSSG), thiobarbituric acid (TBA), taurine, sucrose, and trichloroacetic acid (TCA) were purchased from Sigma (Sigma-Aldrich, St. Louis, MO). Kits for evaluating serum biochemistry were obtained from Pars Azmun (Tehran, Iran). Dimethyl sulfoxide (DMSO), 2-amino-2-hydroxymethyl-propane-1, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), dinitrofluorobenzene (DNFB), 3-diol-hydro-chloride (Tris-HCl), glacial acetic acid, ethylenediaminetetraacetic acid (EDTA), n-butanol, iodoacetic acid, and meta-phosphoric acid were obtained from Merck (Darmstadt, Germany).
Animals
Male Sprague-Dawley rats (200-250 γ weight) were obtained from the Laboratory Animal Breeding Center, Shiraz University of Medical Sciences, Shiraz, Iran. Animals were kept in plastic cages on wood-chip bedding. The ambient temperature was 23 ±1ºC, with ≈40% relative humidity. Animals were allowed to access tap water and a rodent’s pellet chow diet (Behparvar Co., Tehran, Iran). All procedures involving animal use were according to the guidelines for care and use of laboratory animals, which were approved by an institutional ethics committee in Shiraz University of Medical Sciences, Shiraz, Iran (#95-01-36-13830).
Experimental setup and animal surgery
Rats underwent bile duct ligation (BDL) surgery. For this purpose, animals were anesthetized (10 mg/kg of xylazine and 70 mg/kg of ketamine, i.p.), a midline incision was made along the linea alba, and the common bile duct was localized, and doubly ligated (3/0 silk suture). The sham operation consisted of laparotomy and bile duct recognition and manipulation without ligation [46, 47]. Animals were randomly allotted to sham-operated, BDL, and BDL + taurine (50, 100, and 500 mg/kg/day). Tau was administered by intraperitoneal injection for 42 consecutive days.
Serum and urine biochemistry
Rats were anesthetized (thiopental, 50 mg/kg, i.p.), and their blood, liver, and heart samples were collected. Blood was collected from the abdominal vena cava, transferred to standard tubes (Improvacuter; gel and clot activator-coated tubes; Guangzhou, China) and centrifuged (3000 g, 10 min, 4°C) to prepare serum. A Mindray BS-200 autoanalyzer (Mindray chemistry analyzers, Guangzhou, China) and standard kits (Pars Azmun, Tehran, Iran) were used to assess serum biochemistry including alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (γ-GT), creatine kinase-MB (CK-MB), aspartate aminotransferase (AST), and alkaline phosphatase (ALP).
Tissue histopathology
For histopathological assessments, samples of heart and liver tissue were fixed in buffered formalin solution (0.64% sodium phosphate dibasic, 0.4% sodium phosphate monobasic, and 10% formaldehyde in double distilled water; pH = 7.4). Finally, paraffin-embedded sections (5 µm) of tissues were stained with hematoxylin and eosin (H&E). Masson trichrome staining was applied for fibrotic alterations. Tissue slides were blindly analyzed using a light microscope (Olympus CX21, Japan).
Heart tissue reactive oxygen species (ROS) formation
Heart tissue samples (200 mg) were homogenized in 5 ml of Tris-HCl buffer (40 mM, pH = 7.4, 4°C). Afterward, 100 µl of the tissue homogenate was mixed with 1 ml of Tris-HCl buffer (40 mM, pH = 7.4) and 10 µl of 2’,7’-dichlorofluorescein diacetate (final concentration 10 µM) [48-50]. The mixture was incubated in the dark (15 min, 37°C). Finally, the fluorescence intensity was measured using a FLUOstar Omega multi-functional microplate reader (λ excitation = 485 nm and λ emission = 525 nm) [51, 52].
Tissue lipid peroxidation
Thiobarbituric acid reactive substances (TBARS) were measured to assess the amount of lipid peroxidation in the heart tissue of BDL rats [47]. Briefly, 1 ml of heart tissue homogenate (10% w/v in Tris-HCl buffer, 40 mM, pH = 7.4) was added to 3 ml of TBARS assay reaction mixture (1 ml of thiobarbituric acid 0.375%, w/v, 2 ml of trichloroacetic acid 15%; w/v, and 1 ml of hydrochloric acid 12 N, pH = 2). Samples were mixed well and heated in a water bath (100°C, 45 min) [48, 53-55]. Finally, n-butanol (2 ml) was added and vigorously mixed. Samples were centrifuged (3000 g, 10 min), and the absorbance of the developed color in the n-butanol (upper phase) was measured (λ = 532 nm, EPOCH plate reader, BioTek instruments, Highland Park, USA) [56].
Ferric reducing antioxidant power (FRAP) of the heart tissue
The formation of Fe2+-tripyridyltriazine complex (blue colored) from the colorless oxidized Fe3+ was measured by FRAP assay [57]. Briefly, 100 µl of tissue homogenate (10% w/v in Tris-HCl buffer, 40 mM, pH = 7.4) was added to 1 ml of the freshly prepared FRAP reagent (10 volumes of acetate buffer 300 mmol/l, pH = 3.6, 1 volume of TPTZ 10 mmol/l in 40 mmol/l hydrochloric acid 6 N; and 1 volume of ferric chloride 20 mmol/l in deionized water) [58]. Samples were incubated at 37ºC for 5 minutes (protected from light). The absorbance of developed color was measured at λ = 595 nm (EPOCH plate reader, BioTek instruments, Highland Park, USA) [59].
Tissue protein carbonylation
The oxidative damage of proteins was assessed by the determination of carbonyl groups based on the reaction with dinitrophenylhydrazine (DNPH) [60, 61]. Briefly, 100 µl of TCA (20% w/v, 4ºC) was added to 1 ml of the heart tissue homogenate and centrifuged (700 g, 15 min). Then, 500 µl of 10 mM DNPH (dissolved in 2 N HCl) was added to the supernatant. Samples were then incubated for 1 hour in the dark at room temperature with vortexing every 10 minutes. Afterward, 100 µl of TCA (20% w/v) was added, and the tubes were centrifuged (12,000 γ for 5 min). The supernatant was discarded, and the pellet was washed three times with 1 ml of ethanol : ethyl acetate (1 : 1 v/v). The precipitate was re-dissolved in 600 µl of guanidine solution (6 M, with 20 mM potassium phosphate, adjusted to pH = 2.3 with trifluoroacetic acid, for 15 min, 37ºC) and the absorbance at λ = 370 nm was measured (EPOCH plate reader, BioTek instruments, Highland Park, USA) [60].
Heart tissue mitochondria isolation
Rat heart was excised, washed, and minced in an ice-cold (4°C) isolation buffer medium (70 mM mannitol, 2 mM HEPES, 220 mM sucrose, 0.5 mM EGTA and 0.1% BSA dissolved in ion-free double distilled water, pH = 7.4) containing trypsin (0.1% w/v) [62, 63]. Samples were incubated on ice for 15 minutes and centrifuged (15,000 g, 10 min, 4°C). The supernatant was discarded, and the pellet (heart tissue) was transported into the new isolation buffer (10 ml buffer/1 γ of the heart tissue, 4°C). Tissue samples were homogenized, and mitochondria were isolated by differential centrifugation of the heart homogenate [62, 63]. First, unbroken cells and nuclei were pelleted (1000 γ for 20 min at 4°C). Second, the supernatant was centrifuged (10,000 γ for 20 min at 4°C) to pellet the mitochondrial fraction. This step was repeated at least three times using a fresh isolation buffer medium to increase the mitochondrial yield [47, 63, 64]. The mitochondrial fraction pellets (dark brown) were suspended in the incubation buffer containing 225 mM sucrose, 75 mM mannitol, 0.5 mM EGTA, and 2 mM HEPES, pH = 7.4, except for the mitochondrial preparations mitochondrial depolarization, and mitochondrial permeabilization, which were resuspended in mitochondrial depolarization assay buffer (225 mM sucrose, 10 mM KCl, 2 mM MgCl2, 5 mM KH2PO4, 75 mM mannitol, 50 µM EGTA, and 10 mM HEPES, pH = 7.2) and swelling buffer (250 mM sucrose, 2 mM HEPES, 0.5 mM KH2PO4, pH = 7.2) [47]. Samples’ protein concentrations were assessed by the Bradford method to standardize the obtained data [65].
Mitochondrial dehydrogenases activity
Based on a previously described protocol, the reduction of 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide by mitochondrial dehydro-genases was determined [66-68]. Briefly, a mitochondrial suspension (1 mg protein/ml) was incubated with 40 µl of MTT (0.4% w/v, 37°C, 30 min, in the dark). Then, samples were centrifuged (16,000 g, 10 min) and the pellet (product of purple formazan crystals) was dissolved in 1 ml of dimethyl sulfoxide. Finally, the optical density (OD) of samples was measured (EPOCH plate reader, λ = 570 nm, Bio-Tek Instruments, Highland Park, USA) [48, 69].
Mitochondrial depolarization
The mitochondrial capacity to capture the cationic fluorescent dye, rhodamine 123, was used to estimate mitochondrial depolarization [47, 62, 70-74]. Rhodamine 123 accumulates in the mitochondrial matrix by facilitated diffusion. When the mitochondrion is damaged and depolarized, there is no facilitated diffusion. Therefore, the amount of rhodamine 123 in the supernatant is increased [47, 62, 70-73]. In the current study, the mitochondrial fractions (1 mg protein/ml) were incubated with rhodamine 123 (final concentration of 10 µM, 15 min, in the dark, with continuous shaking) [48, 75]. Afterward, samples were centrifuged (16,000 g, 1 min, 4°C), and the fluorescence intensity of the supernatant was monitored (FLUOstar Omega multifunctional microplate reader, BMG Labtech Inc., Germany, λexcitation = 485 nm and λ emission = 525 nm) [62, 76].
Mitochondrial permeabilization
Based on a previously reported protocol, the changes in the light scattering of isolated mitochondria samples at λ = 540 nm was used as an estimate of the mitochondrial swelling and permeabilization [47, 62, 76]. Briefly, isolated heart mitochondria (0.5 mg protein/ml) were suspended in swelling buffer (250 mM sucrose, 2 mM HEPES, 0.5 mM KH2PO4, pH = 7.2) [77]. Then, 100 µl of mitochondrial suspension was transferred to a 96-well plate, and the mitochondrial permeability transition was initiated by adding calcium chloride (final concentration 50 µM). The optical density at λ = 540 nm was monitored for 30 minutes (EPOCH plate reader, Bio-Tek Instruments, Highland Park, USA) [76, 78]. A decrease in the absorbance is associated with an increase in mitochondrial volume and organelle swelling [62, 76].
HPLC analysis of heart tissue and isolated mitochondria glutathione content
The reduced (GSH) and oxidized (GSSG) glutathione levels in the heart tissue and isolated mitochondria samples were assessed by the HPLC analysis of deproteinized samples (TCA 50% w/v) after derivatization with iodoacetic acid and fluoro-2,4-dinitrobenzene, using a 25 cm NH2 column (Bischoff chromatography, Leonberg, Germany), and the flow rate 1 ml/min [79]. The mobile phases consisted of buffer A (water : methanol, 1 : 4 v/v) and buffer B (acetate buffer : methanol, 1 : 4 v/v) and a gradient method with a steady increase of buffer B to 95% in 20 minutes [77, 79, 80]. Nanomole levels of GSH and GSSG can be measured by this method [79]. GSSG and GSH were used as external standards. Heart tissue samples (200 mg) were homogenized in Tris-HCl buffer (250 mM, pH = 7.4, 4°C), and 500 µl of TCA (50% w/v, 4°C) was added. Mitochondria samples (500 µl, 1 mg protein/ml) were also treated with 50 µl of TCA (50% w/v, 4°C) and incubated for 15 minutes on ice. Afterward, samples were mixed well and centrifuged (15,000 g, 15 min, 4°C). Then, 1 ml of the supernatant was collected, and 400 µl of the NaOH : NaHCO3 (2 M : 2 M) was added until the gas production subsided. Afterward, 100 µl of iodoacetic acid (1.5% w/v in water) was added, and samples were incubated for one hour (4°C, in the dark). After the incubation period, 500 µl of 2,4-dinitrofluorobenzene (DNFB, 1.5% w/v in absolute ethanol) was added and incubated in the dark (25°C, at least for 48 hours). Finally, 25 µl of samples were injected into the described HPLC system. The UV detector was fixed at λ = 252 nm [79, 81].
Mitochondrial ATP content
A luciferase-luciferin-based kit (Enliten, Promega, Madison, WI) was used to measure mitochondrial ATP content [82, 83]. Samples and buffer solutions were prepared based on the kit instructions. Briefly, 500 µl of mitochondrial samples (1 mg protein/ml) were treated with 100 µl of ice-cooled TCA solution (0.5% w/v in double-distilled water) and centrifuged (15,000 g, 15 min, 4°C). Afterward, 100 µl of ATP kit content was added to 100 µl of the supernatant (in the dark), and the luminescence intensity of samples was measured (λ = 560 nm using a FLUOstar Omega multifunctional microplate reader, BMG Labtech Inc., Germany) [83].
Lipid peroxidation in heart mitochondria
Thiobarbituric acid reactive substances (TBARS) were measured as an index of lipid peroxidation in isolated heart mitochondria according to a previously reported procedure [62, 76]. Isolated mitochondria were washed to remove sucrose (as a TBARS test interfering agent) using an ice-cold MOPS-KCl buffer (100 mM KCl, 50 mM MOPS, pH = 7.4, 4°C), and re-suspended in fresh MOPS-KCl buffer [62]. Then, the mitochondrial suspension was mixed with twice its volume of a mixture containing trichloroacetic acid (TCA 15% w/v), thiobarbituric acid (TBA 0.375% w/v), 100 µl of 0.24 N HCl, plus 0.5 mM Trolox. Samples were heated for 15 minutes in a water bath (100°C) [62, 84]. After centrifugation (15,000 g, 5 min), the absorbance of the supernatant was measured (λ = 532 nm, EPOCH plate reader, BioTek instruments, Highland Park, USA).
Results
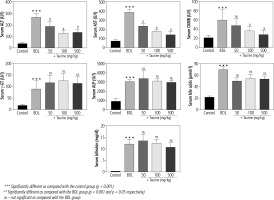
Significant changes in serum biochemical parameters were evident in the BDL group (Fig. 1). Extreme elevation in serum ALP and γ-GT confirmed bile duct injury in the BDL rats. Moreover, serum bilirubin and bile acid levels were significantly higher in the BDL rats. On the other hand, it was found that Tau supplementation (50, 100, and 500 mg/kg) significantly decreased serum biomarkers of organ injury in BDL animals. The effect of Tau on serum markers of organ injury was not dose-dependent in the current study. Moreover, Tau treatment did not significantly change serum levels of γ-GT, ALP, bile acids, or bilirubin (Fig. 1).
Fig. 1
Serum biomarkers of organ injury in bile duct ligated (BDL) rats. Data are given as mean ±SD (n = 8)
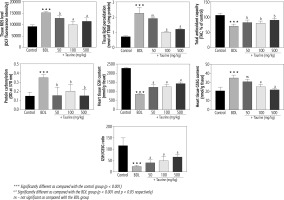
It was found that biomarkers of oxidative stress were significantly increased in the heart tissue of BDL animals (Fig. 2). A significant increase in ROS level, lipid peroxidation, and protein carbonylation, as well as decreased total antioxidant capacity, were detected in the heart tissue of the BDL rats. Significant glutathione depletion, in addition to increased oxidized glutathione (GSSG) level, was also evident in the BDL group. It was found that Tau treatment (50, 100, and 500 mg/kg) significantly ameliorated biomarkers of oxidative stress in the heart tissue of BDL animals (Fig. 2).
Fig. 2
Biomarkers of oxidative stress in the heart tissue of bile duct ligated (BDL) rats. Data are given as mean ±SD (n = 8)
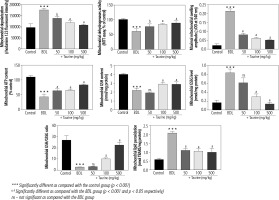
Significant deterioration of mitochondrial indices of functionality was detected in the heart mitochondria isolated from BDL animals (Fig. 3). Mitochondrial depolarization, decreased dehydrogenase activity, mitochondrial permeabilization, lipid peroxidation, and depleted ATP contents were evident in the BDL group. Mitochondria redox balance was also disturbed in BDL animals as the mitochondrial GSH/GSSG ratio was significantly lower. It was found that Tau treatment (50, 100, and 500 mg/kg) positively improved different indices of the heart mitochondria of BDL rats (Fig. 3).
Fig. 3
Mitochondrial indices of functionality in the heart tissue of bile duct ligated (BDL) rats. Data are given as mean ±SD (n = 8)
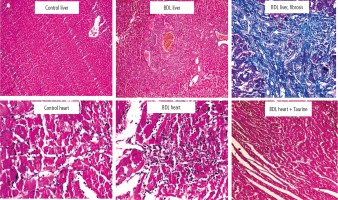
Significant tissue necrosis, inflammatory cell infiltration, and bile duct proliferation, in addition to hepatic fibrotic changes, confirmed the occurrence of cirrhosis in BDL rats (Fig. 4; 42 days after BDL surgery). On the other hand, heart tissue inflammation was the most prominent histopathological alteration in BDL animals. It was found that Tau supplementation (50, 100, and 500 mg/kg) significantly decreased heart tissue inflammation in BDL rats (Fig. 4).
Fig. 4
Rat liver and heart tissue histopathology in control and bile duct ligated (BDL) rats. Significant tissue necrosis, bile duct proliferation, and inflammatory cell infiltration were evident in the liver of BDL animals. Liver histopathological alterations confirmed the occurrence of cirrhosis in the BDL model. Significant tissue inflammation was the most noticeable heart tissue histopathological change in BDL animals. Taurine treatment (50, 100, and 500 mg/kg) decreased heart tissue inflammation in BDL rats
Discussion
It has been well documented that cirrhotic patients suffer from heart injury, arrhythmia, and cardiomyopathy [3, 85]. On the other hand, the precise mechanisms involved in the pathogenesis of this complication remain mostly unknown. There is also no appropriate pharmacological and/or ancillary treatment against cirrhosis-associated cardiac dysfunction [3, 4]. In the current study, it was found that heart tissue biomarkers of oxidative stress were significantly elevated in the BDL model of cirrhosis. Moreover, myocardial mitochondrial function was impaired in cirrhotic animals. It was found that Tau treatment (50, 100, and 500 mg/kg) improved mitochondrial indices of functionality and mitigated oxidative stress in the heart tissue of cirrhotic rats.
The abnormality of heart tissue structure and function has been found in cirrhosis [1, 2, 4, 86]. These effects are known as “cirrhotic cardiomyopathy” [1, 87, 88]. A severe decrease in heart muscle contractility, a decrease in cardiac output, and arrhythmia, as well as portal hypertension, are all linked to heart tissue injury in cirrhotic patients [1, 2, 4, 86, 87]. Cirrhotic cardiomyopathy and heart injury could lead to a series of severe and complicated events which threaten the patient’s life [4, 86].
The histopathological alterations of cardiac tissue in cirrhosis include myocardial hypertrophy, cardiomyocyte edema, exudation, tissue fibrosis, nuclear vacuolation, and unusual pigmentation [88]. Usually, cardiac dysfunction is masked in cirrhotic patients because of “auto-treating” hemodynamic changes (e.g., peripheral vasodilation in cirrhosis) [88]. On the other hand, it has been found that myocardial abnormalities become unmasked after major clinical interventions (e.g., liver transplantation) [88-90]. A high rate of patients’ death has been reported because of congestive heart failure after liver transplantation and other clinical interventions in cirrhosis [88-90]. Therefore, finding the cellular and molecular events leading to cardiac dysfunction in cirrhosis and investigating the protective properties of clinically applicable ancillary treatments could have great value.
Taurine is the most abundant free amino acid in the human body. Tau is considered a conditionally essential amino acid. Several pharmacological effects have been attributed to Tau [91, 92]. On the other hand, many investigations mentioned the potential beneficial effects of Tau in cardiovascular diseases [20, 21]. It has been found that Tau could exhibit regulatory effects on blood pressure [16, 93-96]. Several clinical trials support the view that Tau administration reduces blood pressure in hypertensive patients [16, 93, 94]. Interestingly, this amino acid has been approved for the treatment of congestive heart failure [16]. Physiologically, Tau is found in the heart tissue at very high concentrations (≈25-30 mM) [97]. Taurine transporters (TauT) have been identified in cardiomyocytes [98]. These transporters take up Tau from the systemic circulation and increase myocardial Tau content [98]. On the other hand, it has been found that cardiac function is severely compromised in Tau transporter knockout animals [99, 100]. These data could indicate a role for Tau in cardiac function.
Several mechanisms have been identified for the cytoprotective effects of Tau. It has been well documented that cytoplasmic calcium (Ca2+) level and oxidative stress are modulated by Tau supplementation [101-106]. On the other hand, the effect of Tau on mitochondria is one of the most exciting mechanisms by which this amino acid affects cellular function [16, 27, 38, 103, 107-110]. The regulation of oxidative stress and its associated events is one of the most interesting cytoprotective mechanisms provided by Tau.
Several studies have mentioned that Tau can ameliorate oxidative stress in different pathological conditions [20, 42, 100, 111-113]. Although the mechanisms underlying the antioxidative effects of Tau need more investigations to be precisely clarified, some investigations mentioned the regulatory effects of this amino acid on mitochondrial function as a pivotal mechanism for its antioxidative properties [43, 114-116]. Interestingly, it has been shown that Tau effectively mitigated mitochondria-facilitated ROS formation and enhanced mitochondrial ATP content [12, 36, 37, 117-119]. The protective properties of this amino acid might be mediated through its effect on mitochondria protein synthesis (e.g., mitochondria respiratory chain components) [120, 121]. Tau contributes to the structure of mitochondrial tRNA [43, 122]. It has been found that Tau deficiency impairs proper mitochondrial tRNA function and, finally, protein synthesis [43, 122]. Interestingly, it has also been mentioned that mitochondrial respiratory function and energy metabolism are impaired in the cardiomyocytes of TauT knockout rats [99]. These data could indicate the profound role of Tau in cardiomyocytes. Taurine deficiency in other organs (e.g., in the liver) or deterioration in its trans-porters (TauT) could also impair fundamental physiological processes such as ammonia detoxification in the liver [123].
In the current study, we found that cellular mitochondrial function and energy metabolism were interrupted in the heart tissue of cirrhotic animals. On the other hand, Tau treatment significantly improved mitochondrial function and ATP levels. These data indicate cardiomyocyte mitochondria as a critical therapeutic target for managing cirrhosis-induced heart injury.
Alteration in cardiomyocytes’ lipid bilayer has been documented in cirrhosis experimental animal models as well as human cases [88, 124]. It has been found that the fluidity of the heart tissue plasma membranes is decreased during cirrhosis [88, 124]. Lipid peroxidation and disturbances in the heart tissue membranes might be connected to deleterious events such as electrocardiographic abnormalities in cirrhosis [125]. Stabilizing biomembranes’ lipid bilayer, as well as significant mitigation of lipid peroxidation, are exciting features of Tau [126, 127]. In the current study, we found a high level of TBARs in the heart tissue of cirrhotic animals (Fig. 2). On the other hand, Tau supplementation significantly decreased heart tissue lipid peroxidation in BDL rats (Fig. 2). Therefore, Tau might provide a stable lipid microenvironment in cardiomyocytes of cirrhotic animals.
The effect of Tau on blood pressure has been repeatedly mentioned [128, 129]. Interestingly, it has been found that Tau treatment could effectively decrease portal hypertension in experimental animal models and cirrhotic patients [130, 131]. These data indicate that Tau administration might ameliorate major circulatory disturbances in cirrhosis. On the other hand, the protective effects of Tau on other cirrhosis-associated complications such as hepatic encephalopathy and liver fibrosis have also been documented [132, 133]. Therefore, this amino acid could find application against cirrhosis-associated complications in clinical settings.
Tissue inflammation was the most prominent histopathological alteration in the current BDL animal model of cirrhosis. It has been well established that Tau possesses anti-inflammatory effects in different experimental models [6, 110, 134-136]. Inflammation is an important source of ROS as a massive amount of reactive species, namely superoxide anion, is produced by neutrophils [137, 138]. Therefore, the anti-inflammatory effects of Tau might also play an essential role in its antioxidative and cardioprotective properties in cirrhotic animals.
Proper mitochondrial function in cardiomyocytes guarantees a normal level of ATP that is critical for maintaining cardiac contractility and output. Therefore, decreased cardiomyocytes’ energy (ATP) level in the heart tissue of cirrhotic animals might contribute to cardiac contractile dysfunction and arrhythmia. Surprisingly, there is scarce investigation on the role of mitochondria in the pathogenesis of cirrhosis-associated myocardial dysfunction. The data obtained from the current BDL animal model of cirrhosis indicate that mitochondrial impairment and cellular energy crisis could play a fundamental role in the mechanisms underlying cirrhotic cardiomyopathy and heart failure. Hence, targeting cellular mitochondria could serve as a vital therapeutic point of intervention in cirrhosis-associated cardiac dysfunction (Fig. 5). In the current study, we found that Tau supplementation effectively enhanced cardiac mitochondrial function in cirrhotic animals. Hence, an essential mechanism of cytoprotection provided by Tau might be mediated through preserving mitochondrial function and regulating energy metabolism in cardiomyocytes.
Fig. 5
Cirrhosis-associated complications could proceed to a battery of molecular events in the heart muscle, which finally lead to cardiac dysfunction. Oxidative stress and mitochondrial impairment seem to play a fundamental role in cirrhosis-induced heart injury. Taurine supplementation could regulate mitochondrial function and oxidative stress in the heart tissue of cirrhotic animals.
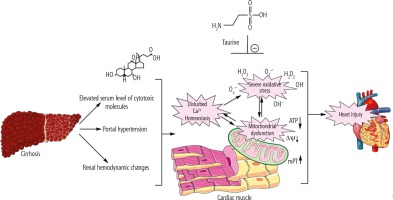
There is no accepted specific treatment for the management of cirrhotic cardiomyopathy. On the other hand, cardiomyocyte mitochondria might serve as potential therapeutic points of intervention in this severe complication. Hence, the administration of antioxidants and mitochondria protecting agents could help in the development of therapeutic strategies against cirrhosis-associated cardiac dysfunction. Tau is an extremely safe amino acid [139]. It has been found that Tau could be administered at very high doses in humans (up to 6 g/day) [20]. Therefore, this amino acid could be clinically applicable as a therapeutic agent in cirrhotic patients.
Conclusions
The data obtained from the current study support the potential cardioprotective properties of Tau in cirrhotic animals. The regulation of cardiomyocytes’ mitochondrial function and mitigation of oxidative stress biomarkers in the cardiac tissue seem to play a significant role in the protective properties of this amino acid. Evaluating the effect of Tau on critical targets such as the renin-angiotensin system and hemodynamic changes could enhance our understanding of the cardioprotective effects of this amino acid in cirrhosis. On the other hand, further studies are warranted to reveal the clinical significance of these data.






