Thermoregulation is defined as the physiological ability to preserve core body temperature independently of ambient temperature The skin has a crucial role in this process through alterations in blood perfusion and evaporative losses [1, 2]. When skin integrity is compromised by a major burn injury, this process is impaired leading to loss of thermoregulation [2].
During the early resuscitation phase, patients with major burns are prone to hypothermia. This is due to a combination of factors including a compromised skin barrier, prolonged exposure of the burnt skin and also as a result of iatrogenic factors such as excessive resuscitation with non-warmed fluids, active cooling to slow the deeper progression of burn injury and pre-hospital anaesthesia [3–5].
A retrospective study demonstrated that hypothermia (< 34.5°C) on admission following a major burn injury was predictive of poor clinical outcomes, notably a longer intensive care unit (ICU) stay and 38% mortality compared to 5% mortality in normothermic patients [6]. Hostler et al. [7] have documented similar findings. Using a logistic regression model they found that hypothermia was associated with mortality independently of other potential clinical confounders [7].
In subsequent days following injury, a profound and dysregulated hypermetabolic response develops, mediated by a 50-fold increase in circulating stress hormones and cytokines and increased sympathetic drive resulting in hyperthermia [8–11]. The resting energy expenditure increases to 40–80% above baseline and correlates with the surface area of burn injury, burn depth and presence of inhalation injury [8–10, 12]. Evidence suggests there is also resetting of the central hypothalamic temperature to around 38.5°C [9, 13, 14]. The hypermetabolic response observed persists for up to 1–2 years after the initial injury [11].
As shown in the study by Wilmore et al. [15], the hypermetabolic response in major burn injury can be mitigated by increasing the room temperature. When the external environment was maintained at a thermal neutral level of 28–33°C, the resting energy expenditure in this group of patients was reduced from 2.0 to 1.4 times the normal requirement [15]. However, there is limited evidence in the published literature on thermoregulation in burns. This is additionally compounded by the variation in perceptions and practice between clinicians involved in day-to-day therapeutic decisions [16, 17].
Attenuation of the hypermetabolic response is also achieved by early excision and grafting of burn tissue, early introduction of appropriate enteral feeding and pharmacological interventions [11, 13, 18]. In addition to modulating the hypermetabolic response, active temperature management is crucial to avoid the negative sequelae associated with hypo- and hyperthermia. Ongoing hyperthermia worsens the existing hypermetabolic state as well as impacting on cellular function, resulting in cell death and multi-organ failure [11, 19]. Hypothermia is associated with increased morbidity and mortality due to coagulation defects and bleeding, increased susceptibility to infection, cardiac arrhythmias and reduced cardiorespiratory function [6, 20–22].
Accordingly, maintenance of temperature appears to be a universal goal throughout all burn units worldwide [23]. However, there is poor consensus over the actual target temperatures. At our Burn Centre, local guidelines recommend maintaining core body temperature within the limits of 38.5 ± 1°C in patients with severe burns, based on an earlier paper by Wilmore [14, 24]. The International Society for Burn Injuries (ISBI) guidelines recommend maintaining the core temperature above or equal to 36°C at all times [23].
The aim of this study was to provide first-hand evidence of practicalities and challenges of the temperature control in adult critically ill patients hospitalised in a tertiary burn centre in relation to local and international guidelines.
METHODS
This study underwent institutional review and approval by the hospital’s Clinical Audit Regi stration System (University Hospitals Birmingham NHS Foundation Trust registration number: CARMS-14835) on 1st of December 2018. The study fulfilled the methodological criteria that permit patient data to be accessed and analysed without the need for written consent. The study included data from Intensive Care patients at the West Midlands Burn Centre (Queen Elizabeth Hospital Birmingham, University Hospitals Birmingham NHS FT, United Kingdom) over an approximately three-year period (1st October 2016 to 7th August 2019). All data were routinely recorded contemporaneously on the hospital’s electronic noting system (PICS System, Birmingham, West Midlands, United Kingdom) and retrospectively analysed.
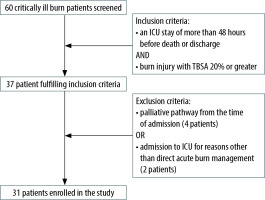
Inclusion criteria were burn injury with total body surface area (TBSA) 20% or greater and an ICU admission of more than 48 hours before death or discharge. Exclusion criteria were patients who were treated palliatively from the time of admission, or patients who were admitted to ICU for reasons other than acute burn management, such as subsequent deterioration or post-operative complications. 31 patients fulfilled the criteria and were included in the study (Figure 1).
Data obtained included patient demogra phics, extent and mechanism of injury, number of surgical procedures, hourly core body temperature measurements during ICU stay, therapeutic interventions used to control temperature and length of ICU stay. These data were collected during initial resuscitation, perioperatively and throughout the ICU stay. Methods of measuring temperature in the ICU include the use of a Foley catheter temperature probe (Philips Medical Systems, Andover, USA), tym-panic thermometer (COVIDien genius 2, Mansfield, USA) and skin temperature probes (Philips Medical Systems, Andover, USA).
Continuous data are presented as a median [interquartile range, IQR], mean (standard deviation, SD) or mean (confidence interval, CI) and analysed using one-way ANOVA test. Categorical data are presented as n (%) and analysed using Fisher exact test. Once collected and categorised, the data were analysed on the cohort level and the individual patient level both for the first 48 hours of admission and across the entire ICU stay. Temperature values were grouped according to four categories: < 36°C (hypothermia), 36–37.4°C, 37.5–39.5°C (local standard) and > 39.5°C (hyperthermia). The impact of TBSA of burn injury and revised Baux (rBaux) score on adequate temperature control was also further investigated using a linear regression model with regression coefficient, 95% confidence interval and P-value reported (Stata Statistical Software 16.1, StataCorp LLC, College Station, TX, USA).
RESULTS
Baseline characteristics
Across the three-year period, a total of 60 patients were admitted to the ICU burns service. 37 patients met the inclusion criteria and 6 were excluded, leaving 31 patients to be studied (Table 1).
TABLE 1
Baseline characteristics of analysed cohort. Mean (SD, range); n (%); median (IQR, range)
Hourly temperature values
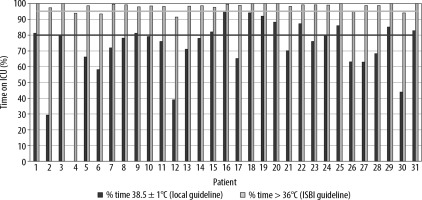
Hourly core body temperatures were collected for each patient during the resuscitation period (defined as the first 48 hours) and the rest of the ICU stay (Figure 2).
FIGURE 2
Hourly temperatures during (A) first 48 hours of the Intensive Care Unit (ICU) admission and (B) whole ICU admission
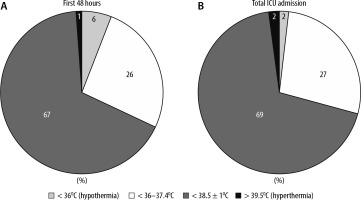
Collectively, 31 patients were cared for in the ICU for a total of 15119 hours.
In the first 48 hours from admission to the ICU, the 31 patients spent a total of 1323 hours on ICU which averaged 43 hours per patient. During the remaining hours, patients were receiving necessary imaging or management of their burn injury in theatre and during this time temperature measurements were not routinely recorded on the electronic system. During these initial hours in ICU, 94% of temperature measurements (1245 hours) achieved the ISBI recommended target (≥ 36°C). Only 78 hours (6%) of temperature values recorded for the cohort were hypothermic (< 36°C). 68% (900 of 1323 hours) of temperature values were within the limits of 38.5 ± 1°C and 1% of temperature values were hyperthermic (> 39.5°C).
During the whole length of ICU stay, hypothermia defined according to ISBI guidelines (< 36°C) was avoided for 14868 hours of ICU care (98% of total stay). The locally agreed targets of body temperature (38.5 ± 1°C) was achieved for 10 417 hours (69% of total stay), although patients body temperature remained between 36.0°C and 37.4°C for 4172 hours (27% of total stay) and hyperthermic (> 39.5°C) for 279 hours (2% of total stay)
Individual patient temperatures
Further analysis was performed to identify if the findings on the overall cohort level were reflective of those for individual patient temperatures.
Considering the ISBI recommendation to maintain body temperature greater than or equal to 36°C, 27 patients (87%) achieved this target for more than 95% of their time in the unit, although only 13 of the 31 patients (42%) had their body temperature maintained within the local aim (38.5 ± 1°C) for more than 80% of their time in the ICU. Ability to maintain temperature within the local standard varied between the patients from 0% to 95% of their stay on ICU (Figure 3).
Maintenance of body temperatures and severity of burns
ANOVA analysis was performed to assess whether there is an association between poor temperature control and severity of burn injury. The patients were divided into three groups according to British Burn Association categories. 12 patients had a TBSA of 20–39%, 10 patients had a TBSA 40–59% and 9 patients had a TBSA ≥ 60%. The results of ANOVA analysis showed that the average time the body temperatures were recorded below 36°C for the above-mentioned groups was 1.25%, 2.1% and 2.1% of ICU stay respectively (P = 0.64). Time within the temperature target of 38.5 ± 1°C was 68.7%, 59.9% and 68.8% (P = 0.75). The differences between the groups were not statistically significant.
Next, we investigated the temperature control of patients and their outcomes. Five of the 31 (16%) patients died during their admission to the ICU.
The five non-survivors had a median ICU admission of 170 hours (range 89–693 hours), median TBSA 45% (range 20–60%) and a median rBaux score 118 (range 82–132). Overall, 24 of 26 (92%) survivors avoided hypothermia, maintaining their temperature above 36°C for more than 95% of their stay in ICU, with only 2 out of 5 non-survivors achieving the same target (P = 0.019). 12 survivors (46%) and only one non-survivor (20%) remained within the limits of 38.5 ± 1°C for the 80% of ICU admission time. The latter difference was not statistically significant (P = 0.37).
Postoperative body temperature
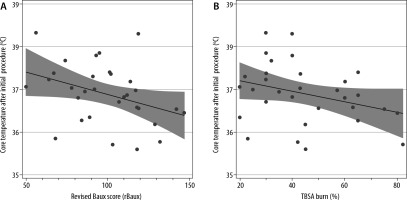
Body temperature is actively controlled in the ICU and operating theatre settings, although intra- operatively patients may be at higher risk of hypothermia. Across the whole patient cohort, the number of theatre episodes varied from 1 to 18. Therefore, the first temperature recordings following return from the first procedure in theatre were analysed. The median post-theatre temperature was 37.0°C within the range of 35.6 to 38.2°C (IQR 36.1–37.9°C). 87% of temperature measurements were above the threshold for hypothermia, although only 16% of post-theatre temperature values achieved 38.5 ± 1°C (Figure 4). No patients were hyperthermic (> 39.5°C) on return from theatre. There was an association between core temperature on the return from theatre and rBaux score with a 0.12°C decrease in post-operative temperature per 10 points increase in rBaux score (β = –0.12, 95% CI: –0.03°C to –0.21°C, P = 0.04). However, the association between TBSA and core temperature on return from the theatre did not reach statistical significance (–0.08, 95% CI: 0.17–0.01, P = 0.08) (Figure 5).
FIGURE 4
Ranges of body temperature following return to the Intensive Care Unit from the operating theatre. 87% of patients were normothermic (≥ 36°C) and the remaining 13% hypothermic (< 36°C). 71% of patients were within the range 38.5 ± 1°C
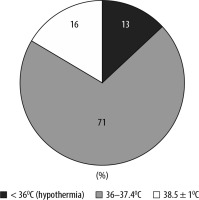
FIGURE 5
Association between (A) revised Baux score (rBaux score) and (B) total body surface area (TBSA) and core body temperature on return from theatre. There was a 0.12°C reduction in temperature for every 10 point increase in rBaux score (β = –0.12, 95% CI: –0.03°C to –0.21°C, P = 0.04). The negative association between TBSA and core body temperature after theatre did not reach statistical significance
Methods of thermoregulation in the ICU
In the ICU, temperature values were most commonly measured using bladder probes. Tympanic thermometers and axillary were the next most commonly used methods however, these were only used in circumstances where the bladder probe failed in the short interim before a new bladder probe could be reinserted or when the patient had clinically stabilised towards the end of their ICU admission and no longer required such strict thermo-regulation control. Hypothermia was predominantly controlled using raised ambient room temperature (90.3%) and forced air warming blankets (80.7%). There were more varied methods to control hyper-thermia. Most common were lowered ambient temperature (80.7%), IV paracetamol (61.3%), removal of external coverings (58.1%), cold bladder irrigation (51.6%) and application of cooled items such as cold face cloths and ice packs (51.6%) (Table 2).
TABLE 2
Methods for control of hypothermia and hyperthermia
DISCUSSION
Temperature control is a significant challenge in the intensive care and perioperative management of patients following a major burn injury. Burn patients exhibit profound hypermetabolism and lose the thermoregulatory function of their skin rendering them susceptible to hypothermia and its associated negative sequelae [5, 11]. Accordingly, it is generally accepted that good temperature control is imperative to achieve good outcomes in major burn patients; however there is little consensus nationally and internationally on target temperatures.
The British Burn Association national standard recommends use of raised ambient temperature in patients with TBSA of injury > 20%, but does not suggest an optimal target temperature [25]. The International Society for Burn Injuries (ISBI) recommends maintaining core body temperature ≥ 36°C [23].
As well as a paucity of guidelines, staff perception and practices also vary among burn centres. A survey investigating practices across 14 UK burns services suggested the perceived threshold for hypothermia and hyperthermia were 36.2°C and 38.8°C respectively [16]. Similarly, Pruskowski et al. [17] conducted a survey across North American burn units to establish perceptions and practices in temperature management of severe burn patients in the ICU and in the operating theatre. Core temperature targets remained within the limits of 36 to 38°C in the operating theatre. In the ICU, targets were based on environmental temperature rather than core body temperature and ranged from 75–95°F (23.9–35.0°C). Interestingly, Pruskowski [17] also comments on the negative effects that ambient temperature has on staff and potential knock on effects on patient care including reduced staff performance and increased risk of sweat contamination intra-operatively. This has been also documented elsewhere [26, 27].
Cohort and individual temperatures
Our results indicate that local and international standards were achieved with 69% and 98% compliance respectively, across the ICU stay. In the first 48 hours of admission (the resuscitation period) this was 68% and 94% respectively. The aim was that the local standard would be achieved 80% of the time and the international standard 95% of the time. The findings suggest that the international goal was achievable across the cohort; however, the stricter local guidance was more challenging to achieve.
Overall, it was equally challenging to control core body temperature between the two time periods analysed (resuscitation period and the whole stay in the ICU). However, critical hypothermia (< 36°C) was slightly more common in the first 48 hours of admission. This is likely owing to fluid resuscitation, frequent operating procedures and prior to application of occlusive dressings and initiation of warming therapies.
On the individual level, there was variability in the ability to maintain core body temperature within the limits of 38.5 ± 1°C. In fact, only 42% of patients maintained their core body temperature 38.5 ± 1°C for > 80% of their ICU admission. The ISBI standard was far more achievable with 87% of patients maintaining their core body temperature ≥ 36°C for > 95% of their time on ICU.
When considering causes for the individual variation in difficulty maintaining core body temperature, our data demonstrated an association between patient outcome and ability to temperature control. 92% of surviving patients avoided hypothermia for more than 95% of their ICU stay, compared to two out of five non-survivors. However, no significant statistical nor clinical association between TBSA and temperature control was identified, when burn severity is grouped according to the British Burn Association categories. These findings differ from other available studies. For example, although focusing on temperatures on admission to a burn centre following injury, Weaver et al. [28] showed that TBSA 20–39% and to a greater extent TBSA > 40% were associated with hypothermia. Their data also showed increasing age, polytrauma and a low GCS were also predictors for hypothermia on admission [28]. Other studies show similar findings [7].
Post-theatre core temperature
Severe burn patients are at higher risk of hypothermia during theatre episodes as a result of prolonged exposure of skin, anaesthetic agents promoting vasodilation and use of intravenous fluids [3, 20, 29]. Hypothermia intraoperatively is associated with increased blood loss, reduced wound healing and infection and therefore efforts should be made to control body temperature perioperatively [21, 29]. This can be achieved by a number of techniques including increased ambient temperature, application of forced air warming blankets, limiting skin exposure and wrapping the head and limbs with insulating materials [20].
In our patient group, the median post-theatre temperature was 37.0°C, with the lowest recorded at 35.6°C, and the highest at 38.2°C. The majority (87%) of post-operative temperature values were ≥ 36°C but only 16% of values achieved the locally recommended target of 38.5 ± 1°C. The findings also suggest that increasing rBaux score is a negative predictor for post-theatre temperature control. It is reassuring that the majority of patients avoid critical hypothermia even after theatre episodes and this demonstrates that the range of techniques used to maintain temperature in our operating theatres are effective.
Measurement and correction of temperature
In the ICU, temperature values were measured using bladder probes. Tympanic thermometers were used in circumstances where the bladder probe failed and, on each occasion, use of a tym-panic thermometer was brief whilst awaiting a new bladder probe insertion. This is in keeping with UK staff perception where bladder thermometer (59%), tympanic thermometers (34%) and oesophageal thermometers (33%) were the most common methods used [16].
Temperature was controlled most using raised ambient temperature (90.3%) as well as warming blankets (80.7%). This is in keeping with survey results suggesting changes in environmental temperature (91%), forced air warming blankets (88%) and warmed intravenous fluids (72%) were most commonly reported methods of managing and avoiding hypothermia in the ICU [16]. Intravascular catheters have been reported for control of both hypo- and hyper thermia in burns patients but are not used in our unit and not widely used within the UK, presumably due to concern of thrombotic complications [16, 30].
A variety of methods were used to manage hyperthermia including lowered ambient temperature (80.7%), paracetamol (61.3%), removal of blankets and dressings (58.1%), application of cold face cloths or ice packs (51.6%) and cold bladder irrigation (51.6%). This again correlates well with surveyed perception where paracetamol (81%), decreasing environmental temperature (76%), haemofiltration (54%), cooled IV fluids (46%) were most commonly reported methods [16].
Limitations
Although we believe this is the largest study to date of temperature control in a cohort of critically ill burns patients, we recognise that this study does have some limitations.
First, recorded temperature measurements in the first 48 hours in ICU do not include the time spent in the pre-hospital or Emergency Department setting where the patient may be more susceptible to hypothermia due to lack of environmental control. Patients are also admitted to the ICU through a variety of pathways; many are transferred from the Emergency Department to theatre and then to ICU thus further increasing the time until ICU temperature measurements can commence and increasing the risk of poor temperature control.
Second, core body temperatures were measured using bladder probes. However, when these failed a tympanic thermometer was used in the interim before a new bladder probe could be reinserted. Although these periods were brief, we acknowledge that it may create a degree of error in the accuracy of core temperature measurement.
Third, towards the end of the ICU admission and as the patients clinically stabilise from their critical illness, less frequent temperature monitoring is required as episodes of critical hypothermia become less frequent. Additionally, the temperature measurements during this period tend to show lower readings because less strict thermoregulation is required at this stage in the patient journey. However, these lower readings may appear to show non-compliance with local guidelines. In any case, avoidance of critical hypothermia should be maintained at all times and so international guidelines should maintain a high level of compliance.
Due to the retrospective nature of the study and relatively limited number of patients, further multi-centre prospective studies would be beneficial to fully address the questions raised by our research, such as the association between severity of thermal injury, inclusive of inhalational element and complexities of temperature management.
CONCLUSIONS
Temperature control is important in patients with major burn injuries who have lost their ability to thermoregulate. They are susceptible to increased morbidity and mortality rates if an appropriate temperature range cannot be achieved. Maintenance of an appropriate core temperature in these patients is challenging.
This study analysed the practicalities of temperature control in the largest known cohort to date of critically ill patients with major burn injuries, measured against both local and international guidelines. We have observed a high variability of temperature control between individual patients, especially in non-survivors, and we have demonstrated an association between high rBaux score and hypothermia, specifically during the perioperative period.
Despite the recognised limitations to this exploratory study, it provides background information for future research into the importance of this subject, determination of appropriate temperature control targets and association with patient outcomes.