Brugada syndrome (BrS) is an ion channelopathy that results in characteristic electrocardiographic (ECG) changes and predisposes to sudden cardiac death (SCD). The SCD risk stratification is influenced by many factors, primarily the ECG pattern and symptoms presented [1]. Asymptomatic patients are considered to be of relatively low risk, especially those with drug-induced Brugada pattern without other risk factors (VF, syncope, etc.) [2]. It is well known that Brugada pattern (BrP) temporal variability is associated with event risk increase (higher temporal of type 1 ST-segment elevation in the 24-hour monitoring period seems to be associated with cardiac events) [3]. However, no cases of BrP variability related to heart rhythm are described.
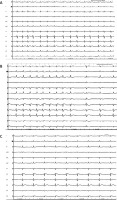
A 68-year-old man was scheduled for paroxysmal atrial fibrillation (AF) ablation (mEHRA score 2b). No drug attempts were made before the procedure, and no family SCD history or syncope were reported in the medical history. We performed AF ablation, under conscious sedation with intermittent midazolam and fentanyl administration, with isolation of pulmonary veins using the CARTO electroanatomic mapping system, Thermocool Smarttouch SF Catheter and Pentaray catheter (Biosense Webster, Inc.). At the end of the procedure, sustained atrial fibrillation was triggered (Figure 1 A). Therefore, having not planned further treatments in the left atrium and having already withdrawn the catheters in the right atrium, we decided to administer flecainide 2 mg/kg over 10 min. After 3 min ECG gradually showed the ST segment coved-type elevation in V1–V2. Then we moved the V5-V6 electrodes to the second intercostal space continuing the infusion according to the protocol. At the end of the infusion, a clear BrP was observed, particularly evident in the V2 derivation shorter RR longer beats, but at arrhythmia interruption, the pattern surprisingly disappeared (Figure 1 B), and then gradually reappeared after 50 s with a diagnostic BrP (Figure 1 C). Based on the patient’s clinical history, the occasional drug-induced BrP finding and considering him to be at low risk of SCD, we decided to evaluate the follow-up without performing an electrophysiological study (EPS). The patient was therefore discharged without antiarrhythmic therapy, with the indication for family electrocardiographic screening and compliance with behavioral rules.
Figure 1
A – Baseline ECG at the end of the procedure; at this time the flecainide protocol started. B – ECG trace showing a gradual coved-type BrP during flecainide infusion was observed. In sinus rhythm BrP disappeared (leads V5–V6 were positioned at the second intercostal space leads V1–V2). C – ECG trace showing the gradual reappearance of coved-type BrP (50 s after sinus rhythm restoration) (leads V5–V6 were positioned at the second intercostal space leads V1–V2)
Several hypotheses have been proposed to explain the pathophysiology behind BrS. The depolarization hypothesis states that there is a conduction delay in the right ventricular outflow tract (RVOT) and it creates an electrical gradient in this part of the heart similar to that associated with myocardial ischemia with the typical ST-segment changes. The repolarization hypothesis considers a genetically driven sodium current alteration to be critical, with an endoepicardial transmural gradient which causes typical ST-segment changes and creates a predisposition for phase 2 re-entry tachyarrhythmias [4].
It is well demonstrated that increased temporal variability in BrP spontaneous manifestation during continuous ICD electrograms monitoring is related to an increased risk of VF [5]. BrP is observed transiently even after electrical cardioversion as a consequence of transmural endo-epicardial gradient [6]. Numerous other causes (metabolic, extracardiac causes, cardiac ischemia, etc.) are also framed in the concept of Brugada phenocopies, without assuming high clinical significance [7].
Our case concerns an occasional finding of a drug-induced BrP during AF pharmacological cardioversion. In particular, we noted that at sinus rhythm restoration the pathological pattern disappeared and then reappeared after 50 s of ECG monitoring. In the literature, several cases of BrP occurrence related to lengthening of the RR interval are reported as a result of ion currents variability and autonomic tone balancing [8]. In other rare cases, physical exertion-related occurrences of BrP have been described, with however uncertain clinical significance [9].
In our case, faster rhythm (not during physical exertion, but during AF) could have increased sodium channel blocking during tachycardia since the diastolic interval becomes too short to let the sodium channels completely recover from the slow inactivated state. However, the phenomenon observed when the arrhythmia is interrupted is unique: lengthening of the RR interval paradoxically modifies the endoepicardial ion currents in such a way that the BrP abruptly disappears. This type of phenomenon is in contrast with what was previously observed in the literature, and we could speculate that the variability of BrP, as previously described, is related to the RR intervals and to the ionic currents but has a stronger link with the autonomic system. In our case, in fact, the restoration of the sinus rhythm probably represents (when the patient was awake) a symptomatic alteration such as exacerbating the adrenergic tone which attenuated the BrP. Only after 50 s was the autonomic balance restored, with the evident reappearance of the BrP. Another possible explanation of the phenomenon may be a sort of drug-induced delay in RVOT intraventricular conduction, which is therefore influenced by the cycle length. This phenomenon would therefore be framed in a sort of drug-induced Brugada phenocopy, which however must focus attention on the use of class Ic drugs due to the possible ability to sustain phase 2 re-entry tachyarrhythmias [4].
It is not easy to give a pathophysiological explanation for the observed electrocardiographic phenomenon; nor can prognostic indications be identified in this particular case. However, the observation of the phenomenon allowed us to adapt the management of any arrhythmic relapses avoiding potentially arrhythmogenic drugs for the patient undergoing catheter ablation.








