Introduction
Breast cancer is one of the most common types of cancer in women. Lifestyle and dietary factors are among the most significant contributors to breast cancer incidence [1]. The human epidermal growth factor receptor 2 (HER-2) signalling pathway has become a promising focus for the development of targeted molecular therapies for cancer. Several chemotherapeutic drugs targeting the HER-2 receptor have been developed, including trastuzumab, pertuzumab, and T-DM1. Trastuzumab is the first monoclonal antibody used clinically for HER-2-positive breast cancer. However, the long-term efficacy of HER-2 therapy remains limited, as approximately 30–70% of patients experience either primary or secondary resistance, which can trigger the development of metastatic disease through the activation of alternative signalling pathways such as PI3K/Akt/mTOR or overexpression of epidermal growth factor receptor [2–5]. In addition, available HER-2 therapies are generally ineffective in targeting the cancer stem cell subpopulation, which plays an important role in tumour recurrence and spread [6]. Combining multiple anti-HER-2 agents may also increase systemic toxicity, financial burden, and clinical complexity without a guaranteed improvement in therapeutic efficacy. Other reported side effects include cardiotoxic disorders and immunological reactions during long-term therapy [7, 8]. Therefore, novel therapeutic agents are needed that not only target the HER-2 and PI3K/mTOR pathways, but also induce oxidative stress and apoptosis, and may be effective against therapy-resistant cancer cells.
Pyrazoline derivatives are nitrogen-containing heterocyclic compounds with diverse biological activities [9–14]. Several pyrazoline derivatives have been investigated for their anticancer properties against various cancer cell lines [15]. These compounds have demonstrated cytotoxic and pro-apoptotic effects in cervical cancer [16, 17], hepatocellular carcinoma [18], lung can- cer [19], and breast cancer cells [20]. Synthetically produced N-phenyl pyrazolines have shown selective anticancer activity, particularly against breast and colorectal cancer cells [17, 21, 22]. Pyrazoline B (3-(2-methoxyphenyl)-2-phenyl- 5-thiophen-2-yl-3,4-dihydropyrazole) is a heterocyclic compound featuring a five-membered ring containing two nitrogen atoms and one double bond (Figure 1).
Our previous studies have shown that pyrazoline B exhibits promising anticancer activity based on both in silico and in vitro approaches [23, 24]. Docking simulations also revealed that pyrazoline B may inhibit HER-2 expression [25]. Therefore, due to its potential to inhibit HER-2 and modulate cell survival signalling pathways, pyrazoline B may serve as a promising therapeutic candidate to overcome the limitations of current HER-2-targeted therapies. In this study, we investigated the cytotoxic and anticancer effects of pyrazoline B on HER-2-overexpressing breast cancer cells using functional cell-based assays.
Material and methods
Pyrazoline B was synthesized and analyzed by Tutik Dwi Wahyuningsih, Ph.D. from the Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Gadjah Mada. The materials used in this study were dimethyl sulfoxide, phosphate buffer saline (PBS), media DMEM (Dulbecco’s Modified Eagle culture Medium), MTT (3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyl tetrazolium bromide) was purchased from Invitrogen, USA, FITC Annexin V (BioLegend), FITC PI3K (BioLegend), PE mTOR (BioLegend), 2’,7’-dichlorofluorescein diacetate (immunochemistry), propidium iodide (BioLegend). The MCF-7/HER-2 cell line was obtained from the Laboratory of Parasitology, Faculty of Medicine, Universitas Gadjah Mada.
Preparation of cells for flow cytometry analysis
The MCF-7/HER-2 cell line was cultured in complete DMEM medium supplemented with 1% foetal bovine serum (Gibco), 1% penicillin-streptomycin (Gibco), and 0.5% fungizone (Gibco). Cells were maintained in a flask at 37°C in a humidified atmosphere containing 5% CO2 [26].
Cell cycle analysis
Cells were fixed in 70% ethanol prepared in PBS for 2 hours at –20°C. After fixation, cells were washed three times with cold PBS, resuspended, and centrifuged at 3000 rpm for 3 minutes. Propidium iodide (PI) staining solution (containing PI at 40 µg/ml and RNase at 100 µg/ml) was added to the pellet, and the mixture was incubated at 37°C for 30 minutes. The samples were analyzed using a FACScan flow cytometer. The proportion of cells in the G2/M phase was determined using ModFit LT 3.0 software based on DNA content analysis [27].
Apoptosis analysis
The Annexin V kit was added to the cell pellet and gently resuspended before incubation at 37°C for 30 minutes. The stained cells were then analysed using a FACScan flow cytometer [28].
PI3K and mTOR analysis
Cells were fixed in cold 70% ethanol in PBS for 2 hours at –20°C. The cells were then washed three times with cold PBS, resuspended, and centrifuged at 3000 rpm for 3 minutes. Permeabilization wash buffer was added to the pellet, followed by resuspension and incubation at 37°C for 10 minutes with PE-conjugated anti-PI3K and FITC-conjugated anti-mTOR antibodies. Samples were subsequently analysed using a flow cytometer [28].
Reactive oxygen species analysis
MCF-7/HER-2 cells (5 × 105 cells/well) were seeded in a 6-well plate and incubated for 24 hours. The cells were stained with 2’,7’-dichlorofluorescein diacetate and incubated for 30 minutes prior to pyrazoline B treatment, followed by an additional 4-hour incubation. Cells were then centrifuged at 2500 rpm for 3 minutes and analysed using a FACScan flow cytometer [29].
Statistical analysis
All data are presented as mean ± standard deviation (SD) from three independent replicates (n = 3). Statistical analysis was performed using GraphPad Prism version 9.0. A one-way analysis of variance was used to evaluate differences among groups. When significant differences were found, Dunnett’s post hoc test was applied to compare each treatment group with the control group. A p-value of < 0.001 was considered statistically significant.
Results
Cytotoxicity activity
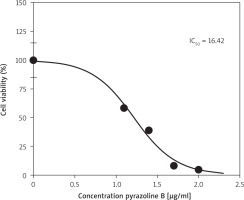
Pyrazoline B was tested for its cytotoxic effect against the MCF-7/HER-2 breast cancer cell line. The compound exhibited an IC50 value of 16.42 µg/ml (Figure 2), calculated based on the percentage of cell growth inhibition.
Cell cycle analysis
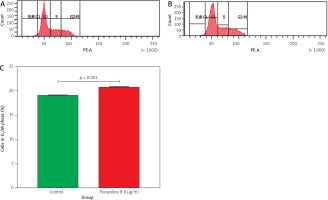
A cell cycle inhibition assay was conducted using pyrazoline B at a concentration of 8 µg/ml. Untreated cells were used as the control group. Flow cytometry analysis was performed to evaluate the effect of pyrazoline B on cell cycle modulation and potential induction of cell death (Figure 3). The results showed that treatment with pyrazoline B increased the proportion of cells in the G2/M phase, from 19.10% in control cells to 20.80% in treated cells (p < 0.001), indicating G2/M phase arrest.
Figure 3
Cell cycle analysis of MCF-7/HER-2 cells after treatment with pyrazoline B (8 µg/ml) for 48 hours. Control cells (A), pyrazoline B-treated cells (B), percentage of cells in G2/M phase (C)
X-axis (A and B): DNA content (PI fluorescence intensity); Y-axis (A and B): cell count (events). Data are presented as mean ± SD (n = 3), p < 0.001 indicates statistical significance.
Apoptosis analysis
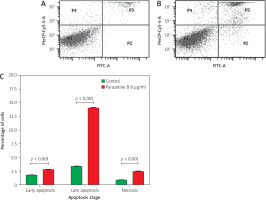
A flow cytometry analysis using Annexin V staining was conducted to evaluate the effect of pyrazoline B on apoptosis induction (Figure 4). The dot plot was divided into four quadrants: P1 (bottom left) represented live cells; P2 (bottom right) indicated early apoptotic cells; P3 (top right) represented late apoptotic or early necrotic cells; and P4 (top left) indicated late necrotic cells. The results showed that treatment with pyrazoline B increased early apoptosis (2.80%), late apoptosis/early necrosis (14.10%), and late necrosis (2.50%) compared to the control group (p < 0.001).
Figure 4
Apoptosis profile of MCF-7/HER-2 cells after treatment with pyrazoline B (8 µg/ml) for 48 hours using Annexin V-FITC/PI staining. Control cells (A), pyrazoline B-treated cells (B), quantification of apoptotic and necrotic cells by quadrant (C)
Quadrants: P1 = viable cells, P2 = early apoptosis, P3 = late apoptosis, P4 = necrosis. X-axis (A and B): Annexin V-FITC fluorescence intensity; Y-axis (A and B): PI fluorescence intensity. Data are presented as mean ± SD (n = 3). p < 0.001 indicates statistical significance.
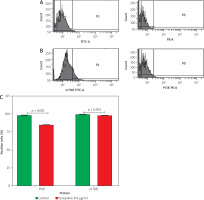
Analysis of PI3K and mTOR expression
Pyrazoline B was evaluated for its ability to reduce PI3K and mTOR expression. MCF-7/HER-2 cells were treated with pyrazoline B for 48 hours, and expression levels of PI3K and mTOR were assessed using flow cytometry (Figure 5). PI3K expression was measured using a FITC-conjugated anti-PI3K antibody. Treatment with pyrazoline B resulted in a decrease in PI3K-positive cells to 85.00%, compared to 98.20% in the control group (p < 0.001). Similarly, mTOR expression was assessed using a PE-conjugated anti-mTOR antibody. Pyrazoline B treatment reduced the proportion of mTOR-positive cells to 98.30%, compared to 99.60% in control cells (p < 0.001).
Figure 5
Flow cytometry analysis of PI3K and mTOR in MCF-7/HER-2 cells after treatment with pyrazoline B (8 µg/ml) for 48 hours. Control cells (A), pyrazoline B-treated cells (B), percentage of PI3K-and mTOR-positive cells (C)
X-axis (A–B): FITC-A (PI3K) or PE-A (mTOR) fluorescence intensity; Y-axis (A–B): cell count (events). Data are presented as mean ± SD, p < 0.001 indicates statistical significance.
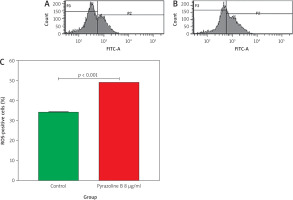
Reactive oxygen species analysis
Flow cytometry was also used to evaluate the effect of pyrazoline B on intracellular reactive oxygen species (ROS) levels. MCF-7/HER-2 cells were treated with pyrazoline B (8 µg/ml) for 48 hours and stained with 2’,7’- dichlorofluorescein diacetate. As shown in Figure 6, treatment with pyrazoline B increased the proportion of cells in the P2 region to 49.20%, compared to 34.20% in the control group (p < 0.001), indicating elevated ROS production.
Figure 6
. Reactive oxygen species production in MCF-7/HER-2 cells after treatment with pyrazoline B (8 µg/ml) for 4 hours, measured using 2’,7’-dichlorofluorescein diacetate staining and flow cytometry. Control cells (A), pyrazoline B-treated cells (B), percentage of reactive oxygen species positive cells in region P2 (C)
X-axis (A–B): 2’,7’-dichlorofluorescein diacetate fluorescence intensity (a.u.); Y-axis (A–B): Cell count (events). Data are presented as mean ± SD (n = 3). p < 0.001 indicates statistical significance.
Discussion
The activity of compounds in inhibiting cell growth is generally classified into three categories: active (IC50 ≤ 20 µg/ml), moderate (IC50 20–100 µg/ml), and inactive (IC50 > 100 µg/ml) [26]. Pyrazoline B demonstrated an IC50 value of 16.42 µg/ml (Figure 2), which falls into the active category, indicating its inhibitory effect on the MCF-7/HER-2 breast cancer cell line. Previous reports have shown that N-phenyl pyrazoline derivatives, such as P2 and P5, exhibited cytotoxic activity against the HeLa cervical cancer cell line, with IC50 values of 20.26 µM and 4.70 µM, respectively [17]. Other pyrazoline compounds have also shown cytotoxic effects against the T47D breast cancer and WiDr colorectal cancer cell lines, with varying degrees of activity and notable selectivity toward cancer cells over normal cells [15]. Due to their broad range of biological activities, particularly anticancer properties, pyrazole and pyrazoline derivatives have been widely recognised as promising scaffolds in drug discovery [30, 31].
Pyrazoline B also promoted cell cycle modulation by increasing the proportion of cells in the G2/M phase (Figure 3), suggesting G2/M phase arrest. Pyrazoline B also promoted cell cycle modulation by increasing the proportion of cells in the G2/M phase (Figure 3), suggesting G2/M phase arrest. This mechanism is consistent with previous findings where an indole-pyrazoline hybrid compound showed IC values as low as 0.034 µM (for HeLa cells) and exhibited low-nM to sub-µM potency in MCF-7, A549 and HepG2 cell lines, and in some cases induced G2/M phase arrest [13]. Likewise, N-formyl pyrazoline derivatives (e.g., 3a and 3l) significantly increased both early and late apoptosis in A549 cells at 4 µM after 24 hours of treatment. The main anticancer mechanism of these compounds involves halting cell cycle progression by targeting specific regulatory proteins, which leads to the accumulation of cancer cells at a particular phase. Such arrest can suppress tumour formation and limit the spread of malignant cells [32].
In addition to cell cycle arrest, pyrazoline B induced various stages of programmed cell death, including early apoptosis, late apoptosis/early necrosis, and late necrosis (Figure 4). Apoptosis and necrosis are critical cellular events in cancer development and treatment. While apoptosis is a genetically regulated process – characterized by DNA damage or fragmentation and mediated by caspases – necrosis typically occurs as an uncontrolled form of cell death. Inhibition of apoptosis is a hallmark of many cancers. Necrosis observed in treated cells may result from accelerated apoptosis progressing to late-stage cell death, particularly under combination treatment conditions [33].
The ability of pyrazoline B to reduce PI3K and mTOR expression after 48 hours of treatment was associated with a decrease in the proportion of marker-positive cells compared to untreated controls (Figure 5). This suggests that pyrazoline B may inhibit or suppress the expression of PI3K and mTOR. Abnormal activation of the PI3K and mTOR signalling pathways is known to play a critical role in carcinogenesis and tumour cell proliferation [34]. Supporting this, previous studies have reported that ferrocenyl pyrazoles (pyrazoline-based compounds) at a concentration of 50 µM suppressed the growth of breast cancer cells (MCF-7 and MDA-MB-231) via inhibition of PI3K/Akt and ERK 1/2 signalling [35].
In this study, the expression of PI3K and mTOR was evaluated by flow cytometry using fluorescently labelled antibodies. Although this technique is useful for quantifying the proportion of cells expressing the proteins of interest, it does not provide information regarding their activation status – such as phosphorylation – or their transcriptional regulation at the mRNA level. This limitation constrains the interpretation of direct pathway inhibition. Recent studies have emphasized that, although intracellular flow cytometry allows for multiparameter analysis, it cannot replace Western blotting for detecting post-translational modifications such as phosphorylation [36]. Therefore, additional validation using methods such as Western blot or quantitative polymerase chain reaction (qPCR) is necessary to confirm the inhibitory effect of pyrazoline B on the PI3K/mTOR signalling pathway [37].
Furthermore, analysis showed that pyrazoline B increased intracellular ROS production (Figure 6). One of the common mechanisms of many chemotherapeutic agents involves the induction of mitochondrial apoptosis via ROS accumulation [38]. Elevated ROS levels can induce cellular senescence and activate anti-apoptotic proteins. Reactive oxygen species are also important in maintaining growth arrest in senescent cells. In some cases, the accumulation of ROS leads to genomic instability and polyploidy, a hallmark of advanced senescence in cancer cells [39]. As reported by previous studies, excessive ROS can cause oxidative damage to proteins, lipids, and nucleic acids, ultimately triggering cell death via apoptosis or necrosis [40]. Therefore, modulating of intracellular ROS levels has been proposed as a promising strategy for cancer therapy [41]. Our findings demonstrate that pyrazoline B inhibits the proliferation of MCF-7/HER-2 breast cancer cells and is associated with increased ROS production, suggesting a possible mechanism involving ROS-mediated apoptosis. This observation aligns with previous findings in the literature [42], where a thiazolyl-substituted bis-pyrazole oxime compound selectively inhibited HCT116 colorectal cancer cell growth through enhanced intracellular ROS. However, the present study did not experimentally confirm whether increased ROS levels were directly responsible for the induction of apoptosis. Thus, further investigation is warranted to establish the causal relationship between ROS elevation and apoptosis in pyrazoline B-treated cells, as has been demonstrated in other studies involving structurally related compounds [43].
Conclusions
The results of this study demonstrated that pyrazoline B exhibits cytotoxic and pro-apoptotic effects on MCF-7/HER-2 breast cancer cells, which are associated with G2/M cell cycle arrest, increased intracellular ROS production, and reduced expression of PI3K and mTOR proteins. These findings suggest that pyrazoline B may exert its anticancer activity by modulating oxidative stress and inhibiting critical oncogenic signalling pathways. However, as the expression of PI3K and mTOR was only assessed by flow cytometry, further studies using Western blot or qPCR are necessary to confirm pathway-level inhibition. Overall, these results indicate that pyrazoline B is a promising candidate for further development as an anticancer therapeutic agent, particularly targeting HER-2-positive breast cancer.