Introduction
Lung cancer is one of the most malignant among cancerous tumours with the highest morbidity and mortality [1]. In recent years, with the enhancement of people’s general health awareness and the application of high-resolution computed tomography (CT) [2], ground-glass opacity (GGO) and solid pulmonary nodules (SPN) can be detected in time. Many of these cases represent early lung cancer or precancerous lesions, where the likelihood of GGO malignancy can be as high as 59–73% [3]. Thoracoscopic surgery is the preferred option for GGO and pulmonary oligometastatic foci. As a type of peripheral pulmonary nodules, subpleural pulmonary nodules are superficial in terms of location. These nodules are small; in particular, the characteristics of GGO foci closely resemble the pulmonary parenchyma and can thus be difficult to detect by finger palpation during surgery [4]. If a subpleural nodule is located at the back of the thorax or at a distance from the surgical incision, even in cases where it is solid or sub-solid, it will be difficult to palpate when using uniportal thoracoscopy. This will result in the prolongation of surgery time, an increase in incisions, conversion to thoracotomy, and even the expansion of the scope of resection. In addition, the complications of a preoperative puncture location can render patients anxious about puncture, leading to a poor perioperative treatment experience. Therefore, accurate localisation and complete resection of pulmonary nodules represent challenging tasks for thoracic surgeons.
Aim
The purpose of this study is to derive the precise positioning and excision of subpleural pulmonary nodules by CT combined with intraoperative percutaneous ultrasonic localisation and to avoid the complications caused by preoperative CT puncture localisation, reduce physiological and psychological stress such as anxiety, the CT radiation dose, and treatment cost, and to improve the treatment satisfaction of patients.
Material and methods
Clinical material
A total of 54 patients with subpleural pulmonary nodules admitted to our hospital from June 2017 to January 2020 were enrolled in the study. All patients were admitted to the hospital for a high-resolution CT scan. Inclusion criteria were as follows: nodules were no further than 10 mm from the pleura. Exclusion criteria were as follows: patients with nodules completely covered by rib or scapula, pleural thickening, or pleural adhesion. Participants were randomly divided into the following groups: 1) a treatment group (n = 23) receiving preoperative CT in combination with intraoperative percutaneous real-time ultrasonic localisation; 2) a control group (n = 31) receiving preoperative CT-guided puncture. Hookwire puncture localisation was adopted in both groups, and U-VATS Pulmonary wedge resection was the preferred option for all nodules.
According to the diagnosis and treatment guidelines for pulmonary nodules, the surgery indications were met and there were no surgery-related contraindications. Participants provided informed consent for their participation in the study prior to undergoing surgery.
Instruments and equipment
High-resolution CT (Somatom GO-UP, Siemens Healthcare, Munich, Germany) was performed with parameters as follows: tube voltage = Sn130 kV, tube current = 56 mA/s, increment collimation = 16 × 0.75 mm, pitch = 1.5, rotation time = 0.5 s. The reconstruction parameters were as follows: slice thickness = 1.5 mm, increment = 1.2 mm, soft-tissue convolution kernel (Br40) and lung convolution kernel (B60), field of view = 400 × 400 mm, matrix: 512 × 512. Ultrasound (Sonoscape Color Doppler ultrasonic diagnosis apparatus) was performed. The ultrasonic probe model was a Canon Convex Array Transducer, model PVT-375BT; Canon Linear Array Transducer, Model PLT-1005BT. Equipment parameters were MI 1.5, 14L5, diffll 10 MHz, 36 fps, G88, DR60. The method for the study involved the use of low frequency (3–5 MHz) for the initial positioning of the lesion, followed by high-frequency (7–13 MHz) line array probe to focus on scanning the area. Additionally, a hookwire with a single-hook puncture positioning needle (20 G*0.7 cm; Gallini Medical Devices) was used.
Material and methods
Preoperative computed tomography body surface location labelling method
High-resolution, CT was used to locate the nodules 1 day prior to surgery or on the day of surgery. According to the surgery position, the patient was placed in a 90° lateral position. The affected upper limb extended towards the head and the hands were laced behind the patient’s head. A body surface positioning grid was placed near the nodule, and the location of the nodule was determined by CT scanning. The nodule located closest to the positioning grid was picked up. The best puncture position was adopted as the intersection point between the positioning grid and the infrared ray and was marked (in blue) on the surface of the body, waiting for the surgery.
Intraoperative ultrasound-guided percutaneous puncture and localisation
Following successful double-lumen endotracheal intubation under general anaesthesia, the patient was placed in the same body position. The pulmonary nodule (red) was again located near the CT positioning point. When the location of the subpleural nodule was displayed on the ultrasound, the hookwire puncture was performed under real-time guidance to localise the nodule within 1 cm, and the needle tip was located 2 cm deep into the nodule. The formation of the pneumothorax was evaluated by ultrasound following the puncture. The external puncture needle was cut through. All patients were operated on by the same sonographer.
Preoperative computer tomography-guided puncture and localisation
On the day of surgery, the patient’s body was properly positioned in the CT room. According to the location of focus, the positioning grid was placed, and a high-resolution CT scan was performed to confirm the puncture point, after confirming the location of the lesion. The distance from the puncture point to the focus and the puncture angle were measured. After routine disinfection, 2% lidocaine was injected to administer infiltration anaesthesia layer by layer. The hookwire puncture needle was inserted within 1 cm around the focus. After confirming the position of the puncture by CT scan, the puncture needle was released and then withdrawn from the puncture sheath. The chest CT was rechecked to confirm the positioning of the puncture needle and whether any complications could be observed, e.g. pneumothorax and haemorrhage. The puncture needle was fixed on the surface of the body, and the puncture point was covered by a sterile application, waiting for the surgery.
Uniportal video-assisted thoracoscopic surgery
For one-lung ventilation, a 3 cm incision was made between the mid-axillary line-anterior axillary line and the fourth or fifth intercostal space, and a 30° thoracoscope was inserted. During surgery, the hookwire positioning needle was found and the body-surface positioning needle was cut off. The lung tissue at the location of the positioned needle was lifted using oval forceps. A straight-line endoscopic stapler was inserted for pulmonary wedge resection at least 2 cm away from the puncture point and needle tip. After removing the pulmonary nodule and local lung tissue, these were removed from the body and packed into a specimen bag. The ex vivo lung tissue was cut along the positioning needle. After discovering the subpleural nodule it was sent for rapid pathology testing; if it was a malignant tumour, the corresponding lobectomy or systemic lymphadenectomy was decided according to the patient’s condition. If it was a benign lesion, a drainage tube was placed and the surgery was completed.
A self-rating anxiety scale (SAS) was used for scoring after completing positioning in the two groups. According to the scale, 50–59 represented mild anxiety, 60–69 represented moderate anxiety, and 70 or above indicated severe anxiety.
Statistical analysis
The age, positioning time, and SAS anxiety scores were compared between the two groups using a t-test. Gender, positioning success rate, and complication rate were compared by χ2 test. Fisher’s precise test was used for positioning nodules; p < 0.05 suggested a statistically significant difference.
Results
The experimental group included seven male and 16 female participants, with an average age of 46.5 ±22.3 years. The distribution of SPN/GGO was as follows: pure GGO (PGGO, a slight increase in the density of shaded or round nodules found in CT. Because the texture of the bronchi and blood vessels inside it can still be displayed, this situation looks very similar to ground glass.), n = 4; mixed GGO (MGGO, a mixed density ground glass opacity, which is the solid components in pGGO with uneven density.), n = 14; and SPN, n = 5. There were 17 cases involving the right lung and 6 cases involving the left lung. The mean size of the nodules was 11.4 ±6.3 mm. The control group included nine males and 22 females with an average age of 50.1 ±18.6 years. The distribution of SPN/GGO was as follows: PGGO, n = 5; mGGO, n = 17; and SPN, n = 9. There were 21 cases involving the right lung and 10 cases involving the left lung. The mean size of the nodules was 10.3 ±4.1 mm (Table I). All patients’ coagulation functioning was normal prior to surgery.
Table I
Comparison of relevant indicators between the two groups of patients
In the treatment group, 22 subpleural pulmonary nodules (22/23) were successfully located (95.6% accuracy), and an average positioning time of 22.0 ±5.9 min was required (Photo 1). The ultrasonic localisation failed in 1 patient at the 6 mm posterior basal segment of the left lower lung, where the pGGO was 9 mm away from the pleura because of complications caused by emphysema. The nodule was found after the remedial positioning in combination with extended cuneiform pneumonectomy. In the control group, 31 patients were positioned successfully (31/31; 100% accuracy) with an average positioning time of 24.2 ±5.4 min. The difference in positioning success rate and positioning time was not statistically significant (p = 0.24; p = 0.15) between the two groups. The total incidence of complications such as pneumothorax, intrapulmonary haemorrhage and unhooking, and SAS anxiety scores in the treatment group were lower than those in the control group, and the difference was statistically significant (p = 0.002; p < 0.00) (Table II). For patients in whom complicated unhooking occurred, the pulmonary nodules were found and removed after re-positioning using a remedial positioning method.
Photo 1
A – Preoperative high-resolution CT showed mGGO of the upper left lung, with a size of 10 × 13 mm (red arrow), a shortest distance skin marker was made (blue dot). B – Percutaneous ultrasound was performed near the CT marker to detect the subpleural nodules (10 mm below visceral pleura, green dot) during the operation, and guide the hook wire to the mGGO. C – CT maker (blue dot) and ultrasonic marker (red dot) have small distance between each other and a good repeatability. D – Hook wire needle was observed under uniportal thoracoscopy, and a pulmonary wedge resection was performed at least 2 cm away from the puncture point. E – Postoperative subpleural nodules were found with naked eye, and pathological indicated infiltrating adenocarcinoma. An extended lobectomy was performed
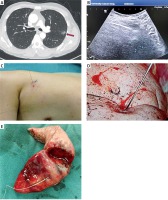
Table II
Comparison of positioning results between two groups of patients
Discussion
This study confirmed that CT-guided noninvasive labelling combined with intraoperative percutaneous ultrasound can be used for positioning of subpleural pulmonary nodules/ground-glass opacity in uniportal video-assisted thoracoscopy. The success rate and time for positioning of the CT combined ultrasound group were comparable with that of the preoperative CT-guided puncture, while the incidence of complications and anxiety scores were lower than the latter.
The popularity of high-resolution CT makes possible early lung cancer diagnosis and treatment and, accordingly, improved prognosis. Thoracoscopic resection is the preferred option for the diagnosis and treatment of pulmonary nodules. Moreover, a uniportal thoracoscope is widely used because it requires a small incision, few cuts, and minor pain. For positioning in the case of subpleural nodules, experienced thoracic surgeons often combine this with CT images, anatomy, and finger palpation. However, in clinical practice, it was found that the smaller the pulmonary nodules, the closer the character to that of lung parenchyma, and the more difficult it was to palpate and locate them. In uniportal thoracoscopic surgery, the surgery area for palpation is additionally limited. If the nodule is far away from the incision or the lung is poorly collapsed, and even when a hypo-solid or solid nodule is below the pleura, it can be difficult to palpate using fingers, whereas the GGO can be almost impossible to locate, even in ex vivo lung tissue [5]. A hybrid operating room and electromagnetic navigational positioning technology [6, 7] present significant technical requirements for doctors, as well as extended surgery times. The equipment involved in these contexts is expensive and often takes a long time to operate in the surgical theatre. As such, it is not conducive to extensive promotion.
At present, imbedding of the hookwire, micro spring coil, and using methylene blue and medical glue are typically employed for preoperative CT positioning [8]. Preoperative puncture positioning is an invasive procedure during which patients must bear complications such as pneumothorax, pleural reaction, and bleeding (lung and chest wall) caused by local anaesthesia puncture, physiological suffering such as pain and limitations to breathing and motion, and psychological burdens such as tension and anxiety [7]. In the present study, the incidence of unhooking and pneumothorax in the control group was significantly higher compared with the treatment group. It was assumed that patients’ breathing, coughing, and thorax movement following the puncture led to the loosening of the puncture needle and secondary lung injury. In the treatment group, the primary nodule localisation (localisation only, no puncture) was affected using only one CT scan. Because no anaesthesia and puncture procedures were completed prior to surgery, no complications occurred. Patients were able to easily cooperate with the procedure and had to endure only a short positioning time, and low scanning dose and cost. The positioning time could be flexibly arranged on the day of the procedure or the day prior to the procedure, without immediate surgery following positioning. Because the puncture was performed after effecting general anaesthesia, there was no pain throughout the entire procedure, even when complications occurred, and no risk of positioning failure caused by unhooking. As a result, the anxiety scores for the positioning procedure were significantly lower compared with the control group. The reduction of overall complications reflected the advantage of this essentially noninvasive approach, which was more acceptable to the patients.
Ultrasound is a method that can accurately estimate the shape, edge, echo, blood flow, acoustic shadow, and compressibility of specific lesions. Research results [9–12] showed that the effect of ultrasound-guided real-time biopsy of lung lesions close to the pleura, or within 10 mm of the pleura, was the same as for CT-guided biopsy. The procedure includes advantages such as good safety and low cost. Japanese scholars [13] found that in the case of lung tissue collapse, an intraluminal ultrasound can find the lung tissue structure within 30 mm of the pleura and can locate the pure GGO at a diameter less than 20 mm. Hironobu [14] selected pig lungs, rabbits, and other animal models as research objects. In the case of atelectasis, a special thoracoscopic ultrasound probe was able to accurately locate the sub-centimetre pulmonary nodules, where the average nodule size was 8.5 ±2.1 mm and the mean depth was 7.4 ±7.5 mm. This evidence indicates that if there is no obvious gas interference on the surface of pulmonary nodules, it is feasible for the subpleural nodules or even the GGO to be located by percutaneous ultrasound. Japanese scholars [15] located pulmonary nodules using the vertical CT-labelling method on the surface of the body under the “pulmonary functional residual capacity time phase” and achieved good results. However, because the present study did not apply real-time guided positioning, the positioning was at times uncertain and not precise. The difference in positioning success rate was not statistically significant between the two groups in the present study because the reserved CT positioning points reduced the scope of ultrasound localisation, which enabled the intraoperative percutaneous ultrasound to quickly discover the subpleural nodules. Furthermore, the puncture needle positioning was guided in real-time, thereby avoiding blindness and the risk of unhooking the intraoperative simple percutaneous ultrasound localisation, improving the positioning success rate of the GGO and shortening positioning and anaesthesia administration times. These results indicate the potential advantages of a noninvasive positioning method.
This study had some limitations. Positioning failed for 1 patient due to the deep pGGO position in this case and complications related to COPD, resulting in pneumothorax interference on the nodule surface. This method should thus be carefully applied for patients with deep nodules and emphysema. In addition, the ultrasound positioning time in the case of small pGGOs is relatively long and requires an experienced sonographer who can patiently perform surgery to reduce the likelihood of failure. A follow-up study will increase the sample size and explore the accuracy of this method in cases of positioning for deeper and smaller subpleural nodules.
Conclusions
It is feasible to locate subpleural nodules by CT-guided noninvasive labelling in combination with intraoperative percutaneous ultrasonic puncture and localisation, with the advantages of high accuracy, non-invasive, safe, low cost and patient have a good tolerance, among others. It is thus worth applying in uniportal video-assisted thoracoscopic surgery.









