Introduction
Ovarian cancer (OC) accounts for 6% of all deaths among women in Poland with per annum figures of more than 2000 mortalities and more than 3500 new cases [1]. The 5-year survival rate is approximately 47.6%. The majority of women with OC (60%) already have an advanced stage of the disease at the time of presentation; and in those cases the 5-year survival rate is only 29% [2, 3]. Nowadays, cytoreductive surgery is a mainstay of therapy for OC, followed by chemotherapy. A key factor in the patient’s survival prognosis is “complete” cytoreductive surgery that achieves complete clearance of all macroscopic neoplastic implants, including gross lymph node metastases [4, 5].
According to the current guidelines of the International Federation of Gynecology and Obstetrics (FIGO) and the European Society of Gynaecological Oncology, the removal of both pelvic and paraaortic lymph nodes during surgery is still a recommended procedure for apparent early stage (FIGO I and II) epithelial OCs [6–8]. In cases of advanced OC (FIGO stages III and IV), Harter et al. have shown, in their Lymphadenectomy in Ovarian Neoplasms (LION) study, that the systematic lymphadenectomy of clinically unchanged lymph nodes in patients with advanced OC does not have a positive influence on patients’ overall survival (OS). In those cases, the complete resection of unchanged lymph nodes should be avoided to reduce post-operative morbidity and mortality [9]. On the other hand, enlarged, metastatic lymph nodes should be precisely resected to keep the maximum possible cytoreductive completeness, even at the risk of morbidity connected with the extent of the surgery increasing significantly.
The LION study revealed that as many as 55.7% of patients with advanced OC presented with lymph node micrometastases. However, this type of lymph node spread had no impact on patient survival. Still, as indicated by current clinical guidelines and by the LION study, the removal of enlarged, metastatic lymph nodes is an important part of cytoreductive surgery for OC [7, 9]. Nevertheless, the exact impact of lymph node macrometastases on OC patient survival is not precisely defined [9, 10]. Therefore, the idea behind our study was to evaluate what information can be gained by analyzing lymph node metastases in OC patients. Additionally, we planned to clarify the impact of lymph node metastases in relationship to FIGO staging of the diseases and residual disease after surgery.
The main aim of our study was to analyze the relationship between the presence, number and types of lymph node metastases and OC patient prognosis. We presumed that analysis of the numbers and of lymph node metastatic involvement could enable the development of a more individualized approach across a range of surgical treatments in patients with OC [10, 11].
Material and methods
Data were collected retrospectively from the databases of three institutions between 2010 and 2015: the Clinical Department of Gynecological Oncology, Franciszek Lukaszczyk Oncological Center, Bydgoszcz, Poland; 1st Department of Obstetrics and Gynaecology, Centre of Postgraduate Medical Education, the Independent Public Clinical Hospital of Professor W. Orłowski in Warsaw; and the 2nd Department of Obstetrics and Gynaecology, Centre of Postgraduate Medical Education, Bielański Hospital, Warsaw, Poland. Patient demographics, treatment details, pathological details and follow up details were obtained from a prospective database. Patients with borderline tumors and non-epithelial ovarian tumors were excluded. We included only epithelial OC patients who had undergone surgical treatment during primary or interval debulking surgery. Patients underwent longitudinal laparotomy extending from the xiphoid process to the pubic bone. All the patients had received a bilateral/unilateral salpingo-oophorectomy and pelvic peritonectomy with retroperitoneal hysterectomy or, in the case of a previous hysterectomy, vaginal vault resection. Additionally, total omentectomy had been performed. Other procedures, such as diaphragmatic peritonectomy, splenectomy, resection of liver metastases, or bowel resections, had been performed, when necessary, depending on the degree of tumor infiltration, in order to remove all macroscopic lesions. Complete pelvic and paraaortic lymphadenectomy, up to the level of renal veins, had been performed as part of debulking surgery following FIGO guidelines [8]. All procedures were performed by accredited gynecological oncologists (in most cases, LW and GP). The final histopathological diagnosis was made and the tumors were classified according to World Health Organization (WHO) guidelines [12]. Disease staging was then assessed using the FIGO classifications from 2014 [13]. Cases treated prior to 2014 were reclassified using the 2014 classifications. All patients had received intravenous antibiotic prophylaxis composed of first-generation cephalosporin. Some patients had received postoperative parenteral nutrition according to the extent of their surgery and nutritional status. Transfusions of red blood cell concentrates (RCCs) were administered depending on patients’ clinical performance; however, most patients with postoperative hemoglobin concentrations below 8 d/dl received RCCs. Except for cases of early mortality, all patients had received first-line chemotherapy consisting of intravenous carboplatin and paclitaxel.
We analyzed duration of surgery, number of days between surgery and chemotherapy treatment, and number of patients after primary cytoreductive surgery and interval cytoreductive surgery. We analyzed OS from the day of each patient’s surgery until their death and made comparisons between 3 groups of patients: those after lymphadenectomy without lymph nodes metastases, those after lymphadenectomy with lymph nodes metastases and those without lymphadenectomy. We also compared patient survival rates according to the completeness of their cytoreductive surgery (according to the Sugarbaker score [14]) and the FIGO stages of disease in the same three groups identified above.
In the group of patients with metastatic lymph nodes we analyzed the impact on OS of: the number of metastatic lymph nodes, the lymph node ratio (LNR), the lymph node metastasis depending on the histopathological type of epithelial OC (EOC) and the extracapsular involvement.
We analyzed the impact of LNR on patient OS. The LNR was defined as the ratio of the number of metastatic (positive) lymph nodes to the total number of resected lymph nodes. To evaluate the impact of LNR we performed a multivariate survival analysis using Cox proportional-hazards regression with the stepwise entry method; and we analyzed the LNR cut-offs incrementally at 0.05 intervals, starting at 0.05, and then at 0.1, 0.15, 0.2, and so on, up to and including 0.95.
The nonparametric Mann-Whitney test was used to compare the study groups with respect to patient age and duration of surgery. The analysis of FIGO stage distribution was performed using the χ2 test. Information on any patients who died was retrieved from the database of the National Health System of Poland. Survival analyses were conducted using the Kaplan-Meier survival curves and the differences in patient survival were compared using the log-rank test. Multivariate survival analysis was conducted using Cox proportional-hazards regression with the stepwise entry method. Statistical analysis was carried out using MedCalc 11.4.2.0 and GraphPad InStat 3.06.
The study was approved by the Centre of Postgraduate Medical Education Ethics Committee (8/PB/2020).
Patients’ characteristics
We identified 801 patients who had previously undergone surgery for OC. We excluded patients with borderline tumors or nonepithelial tumors, and patients with an incomplete medical history. Finally, we evaluated 651 patients. Cytoreductive surgery had been performed in all included patients.
We divided our study population into three sub-groups according to their lymphadenectomy status: patients with lymphadenectomy and without metastases to the lymph nodes (n = 274; 42%), patients with metastases to the lymph nodes following lymphadenectomy (n = 144; 22%) and patients who did not have a lymphadenectomy during their cytoreductive surgery (n = 233; 36%). The median number of lymph node metastases was 4 (range 1–32). We observed significant variations in the FIGO stages, the most common FIGO stage being III (n = 459; 70.5%); and we observed significant differences in the distribution of histopathological types between the three subgroups. The most common histopathological type of neoplasm in all three groups was serous OC (n = 485; 74.5%). Across the groups, patients did not differ from each other in the median time to first chemotherapy. Patients who had no lymphadenectomy had significantly shorter surgery duration. Table 1 summarizes the detailed characteristics of those patients included in the study.
Table 1
Characteristics of patients with ovarian cancer included in the study
The highest number of lymph node metastases was found in serous OC patients (25.3%); lower numbers were found in patients with mucinous OC (17%) and clear cell OC (14%); and the lowest of all was in the group of endometroid OC patients (6.5%).
Survival analysis
In our study group, the patients who had lymphadenectomy during cytoreductive surgery and in whom no lymph node metastases were found (n = 274) had a significantly higher median overall survival (mOS) (2304 days – d), range 0–3178) compared with both those patients without lymphadenectomy (n = 233, mOS 540 d, range 1–3165) and those patients with confirmed lymph node metastases (n = 144, mOS 754 d, range 1–2219, p < 0.001, Fig. 1A). Similar results were observed when the survival analysis was conducted separately, based on residual disease after surgery (Fig. 1B–1D), on the FIGO stage of the disease (Fig. 1E–1L) and on the histopathological type of the tumor (Fig. 2). However, in the case of patients with minimal residual disease (CC1), the difference in patient OS was not related to either lymphadenectomy or the presence of lymph node metastases. Similarly, the differences were not significant in the cases of FIGO stage I, stage IIIA, stage IIIB, stage IVA and stage IVB patients (Fig. 1).
Fig. 1
Survival analyses according to lymphadenectomy in patients operated due to ovarian cancer. Group 0: patients without lymphadenectomy, group 1: patients after lymphadenectomy without metastases, group 2: patients after lymphadenectomy with metastases to the lymph nodes. A) Group 0: n = 233, median overall survival (mOS) 540 days (d) (range 1–3165) vs. group 1: n = 274, mOS 2304 d (0–3178) vs. group 2: n = 144; mOS 754 d (1–2219). B) Group 0: n = 84, mOS 726 d (1–3165) vs. group 1: n = 220, mOS not reached (NR): (12–3178) vs. group 2: n = 93; mOS 1125 d (1–2219). C) Group 0: n = 36, mOS 608 d (2–1765) vs. group 1: n = 23, mOS 1028 d (0–2407) vs. group 2: n = 22, mOS 573 d (38–1469). D) Group 0: n = 113, mOS 381 d (1–2818) vs. group 1: n = 33, mOS 2693 d (0–3114) vs. group 2: n = 28, mOS 520 d (52–2065). E) Group 0: n = 12, mOS NR (190–3163) vs. group 1: n = 65, mOS NR (30–3178). F) Group 0: n = 22, mOS 708 d (1–2793) vs. group 1: n = 47, mOS NR (148–3177). G) Group 0: n = 172, mOS 540 d (1–3165) vs. group 1: n = 154, mOS 1770 d (0–3155) vs. group 2: n = 118, mOS 744 d (0–2219). H) OS patients after lymphadenectomy in subgroups IIIA: group 0: IIIA1(i); n = 8, mOS NR (327–1871) vs. group 1: IIIA1(ii); n = 12, mOS 1228 d (287–1819) vs. group 2: IIIA2; n = 10, mOS NR (169–2163). I) Group 0: n = 7, mOS 1256 d (265–1758) vs. group 1: n = 7, mOS 895 (108–1770) vs. group 2: n = 14, mOS 381 (87–925). J) Group 0: n = 155, mOS 580 d (0–1877) vs. group 1: n = 125, mOS 1539 (0–3155) vs. group 2: n = 98, mOS 651 (1–1668). K) Group 0: n = 26, mOS 152 d (6–1905) vs. group 1: n = 9, mOS 846 d (267–2319) vs. group 2: n = 11; mOS 539 d (52–2039). L) Group 1: patients with FIGO IVA after lymphadenectomy (L) without metastases to lymph nodes (LN); n = 9, mOS 368 d (13– 835) vs. group 2: patients with FIGO IVA after L with metastases to LN; n = 3, mOS 51 d (17–449) vs. group 3: patients with FIGO IVA without L; n = 5, mOS 53 d (0–263) vs. group 4: patients with FIGO IVB after L without metastases to LN; n = 8, mOS 409 d (267– 1106) vs. group 5: patients with FIGO IVB after L with metastases to LN; n = 11, mOS 730 d (5–2319) vs. group 6: patients with FIGO IVB without L; n = 9, mOS 539 d (12–1905)
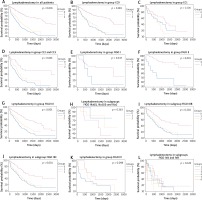
Fig. 2
Survival analyses according to lymphadenectomy and metastatic lymph nodes in different histopathological types of EOC. Group 0: Patients without lymphadenectomy, group 1: patients after lymphadenectomy without metastases, group 2: patients after lymphadenectomy with metastases to the lymph nodes. A) Group 0: n = 175, mOS 597 d (1–3165) vs. group 1: n = 178; mOS 2101 d (0–3178) vs. group 2: n = 124, mOS 784 d (0–2127). B) Group 0: n = 26, mOS 298 d (1–3034) vs. group 1: n = 43, mOS NR (18–3150) vs. group 2: n = 14, mOS 499 d (14–499). C) Group 0: n = 12, mOS 574 d (45–2821) vs. group 1: n = 31, mOS NR (0–3155) vs. group 2: n = 3, mOS NR (495–2008). D) Group 0: n = 17, mOS 268 d (1–799) vs. group 1: n = 19, mOS 1770 d (29–2199) vs. group 2: n = 6, mOS 798 d (76–1282)
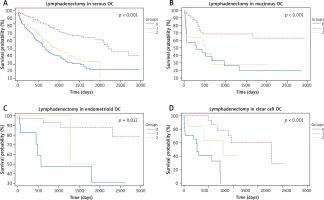
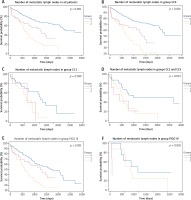
Within the group of patients with metastatic lymph nodes, we observed significantly shortened OS (p < 0.001) in patients (n = 78) where metastases had spread to 4 or more lymph nodes (mOS 587 d; range 1–2219) when compared with patients (n = 276) with lymphadenectomy and negative lymph nodes (mOS 2304 d; range 1–3178) and patients (n = 65) with metastases in 1 to 3 lymph nodes (mOS 1247 d, range 0–2091) (Fig. 3A). The results were confirmed when the number of lymph node metastases was analyzed in relationship to the FIGO staging of the disease and the residual diseases after surgery (except for CC1, where no significant difference was observed; Fig. 3B–3F).
Fig. 3
Group 0: patients after lymphadenectomy without metastases, group 1: patients after lymphadenectomy with 1–3 metastases to the lymph nodes, group 2: patients after lymphadenectomy with more than 4 metastases to the lymph nodes. A) Group 0: n = 276, mOS 2304 d (1–3178) vs. group 1: n = 65, mOS 1247 d (0–2091) vs. group 2: n = 78; mOS 587 d (1–2219). B) Group 0: n = 220, mOS NR (12–3178) vs. group 1: n = 53, mOS 1316 d (0–2091) vs. group 2: n = 40; mOS 798 d (1–2219). C) Group 0: n = 23, mOS 1028 d (0–2407) vs. group 1: n = 5, mOS 573 d (63–820) vs. group 2: n = 17; mOS 544 d (38–1469). D) Group 0: n = 33, mOS 2693 d (0–3135) vs. group 1: n = 7, mOS 404 d (65–1394) vs. group 2: n = 21; mOS 520 d (76–2065). E) Group 0: n = 154, mOS 1770 d (0–3155) vs. group 1: n = 62, mOS 1247 d (0–2091) vs. group 2: n = 70; mOS 587 d (1–2219). F) Group 0: n = 9, mOS 846 d (267–2319) vs. group 1: n = 3, mOS 62 d (42–258) vs. group 2: n = 8; mOS 617 d (61–2039)
In the group of patients with lymph node metastases, the multivariant survival analysis indicated 0.1 as the best cut-off for LNR to differentiate between short- and long-term survivors, with a hazard ratio of 3.1 (95% CI 2.13–4.52). Patients with LNR ≥ 0.1 (n = 112) had significantly shorter OS (578 d, range 1 –2219) compared with patients with LNR < 0.1 (n = 298), whose mOS was 2304 d (range 52–3170, p < 0.001). Similar results were found when we analyzed patients in groups FIGO III and FIGO IV, and CC0 and CC1. Detailed results are presented in Figure 4A–4C.
Fig. 4
Survival analyses according to the lymph node ratio (LNR) and extracapsular spread. LNR is defined as the ratio of the number of metastatic lymph nodes to the total number of resected lymph nodes. Group 0: patients after lymphadenectomy with LNR < 0.1, group 1: patients after lymphadenectomy with LNR > 0.1. A) In all patients; group 0: n = 298, mOS 2304 d (0–3178) vs. group 1: n = 112, mOS 578 d (1–2219). B) Group 0: n = 32, mOS 803 d (0–3114) vs. group 1: n = 26, mOS 520 d (52–1394). C) Group 0: n = 155, mOS 1600 d (12–2407) vs. group 1: n = 86, mOS 744 d (1–2219). D) Survival analyses according to extracapsular spread (ECS) in metastatic lymph nodes A. Group 0: patients after lymphadenectomy without ECS in metastatic lymph nodes: n = 104, mOS 729 d (0–2219), group 1: patients after lymphadenectomy with ECS in the metastatic lymph nodes: n = 38; mOS 958 d (1–2039)
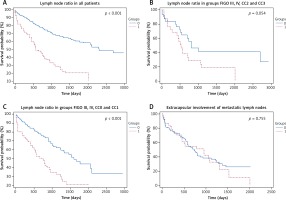
Regarding the extracapsular spread (ECS) of carcinomatosis in resected lymph nodes, patients with confirmed ECS (n = 38) had a shorter (mOS: 958 d, range 1–2039) than those patients without ECS in lymph node metastases (n = 104) without ECS (mOS 729 d, range 1–3178; p < 0.755). Patients with ECS also exhibited more advanced stages of disease, a larger number of positive lymph nodes (PLN). The results are summarized in Figure 4D and in Table 2.
Table 2
Characteristics of patients with extracapsular spread (ECS) of carcinomatosis in resected lymph nodes
We performed a multivariant survival analysis to evaluate the impact of lymph node metastases. The analysis evaluating the impact of the number of lymph node metastases (none vs. 1–3 vs. 4 and above), LNR (at the cut-off ≥ 0.1), FIGO stages of the disease, residual disease using Sugarbaker scoring, and extracapsular involvement, found that the only independent predictors of patient OS were: FIGO stage of the disease (p = 0.005), residual disease (p = 0.041) and LNR (p < 0.001).
Discussion
The main results of our study indicate that the presence of lymph node metastases in OC is a negative predictor of patient survival. The presence of lymph node metastases and increased LNR were also associated with poor survival rates when the groups were analyzed according to the FIGO stages of the disease and the extent of residual disease. We especially found that LNR equal to and above 0.1 was an independent predictor of poor survival.
Ataseven et al. [15] analyzed 809 patients operated on because of advanced EOC and estimated an LNR of 0.25 as an optimal prognostic cut-off value for more precisely predicting overall survival than using the conventional method of lymph node status in EOC patients. Another similar analysis was conducted [16] on the importance of LNR in advanced clear cell OC, and this study also reported that patients with an elevated LNR > 0.25 had worse progression-free survival and OS than patients with LNR. In our study the LNR cut-off with the greatest predictive value was LNR < 0.1 to differentiate short- and long-term survivors. Tong et al. [17] estimated the metastatic LNR and showed that an LNR cut-off of 42% was an optimal predictor for clinical prognosis of EOC patients in an advanced stage of the disease. Patients with LNR > 42% had significantly poorer OS, independent of age (< 60 years, > 60 years) or FIGO stage (FIGO III or FIGO IV). Moreover, the Tong et al. study concluded that LNR is a better predictor than using the number of PLN.
We observed that when there are more than 4 metastatic lymph nodes, this incidence is a significant predictor of a worse prognosis for patients with OC after cytoreductive surgery, and their overall survival rate is conclusively shorter. As with our data, Tong et al. [17] found that mortality risk decreased in correlation with an increase in the number of PLN, examined in three groups (1 PLN, 2–4 PLN, and > 4 PLN). Further, the study by Mahdi et al. [18] showed that a value greater than 3 PLN is an independent predictive factor in a poor prognosis for patients with clear cell OC aside from the number of lymph nodes removed (comparing groups with < 10 removed and > 10 removed) [18]. However, the number of PLN is dependent on many factors, such as the surgeon’s and pathologist’s experience, anatomical variation, or the extent of the tumor [19], and this PLN figure does not provide or infer any information about the number of negative lymph nodes [20]. It is why the LNR value has been found in several studies to be a more accurate predictor [15, 17, 20]. Our study indicated similar results; for instance, in our multivariate survival analysis, the LNR was the only significant predictor of patient OS, while the number of lymph node metastases and the extracapsular involvement were not found to be independent factors. Other research, directed by Wang et al. [20], has also shown that an increasing number of resected lymph nodes correlated with a significant improvement in OS for FIGO stage II and III cases of the disease. However, for FIGO stage IV patients, an improved OS was not significantly associated with a more extensive lymphadenectomy. These observations were also corroborated by our analysis, because lymphadenectomy and the presence of lymph node metastases were not associated with patient OS among patients with FIGO stage IV EOC.
ECS has been shown to be a negative predictor of patient survival in numerous neoplasms, including cervical, breast, gastric, and head and neck cancers [21–25]. In our study, we found the ECS of metastasis in lymph nodes in 26% of cases, and this result is comparable with Heublein et al.’s [26] analysis. However, in that study, the extracapsular spread of metastasis in the lymph nodes in 29% of cases was an important indicator of tumor aggressiveness and a negative prognostic factor for overall survival in patients with advanced OC [26]. In our group, ECS was associated with an advanced stage of the disease, blood transfusion rates, and a longer interval between the first course of chemotherapy treatment and surgery; however, we did not find an association of ECS with shortened OS. In Heublein et al.’s analysis, there were no significant correlations with patient age, tumor size, residual disease or the number of dissected nodes, which can compare with our results [26].
The presence of lymph node metastases in various histopathological types of OC is distinct. Most of the studies, including ours, indicate that high grade serous OC is associated with higher incidences of lymph node metastases compared with other types of EOC. We found that 25% of patients with serous OC we studied had lymph node metastases. Similar results were obtained by Zhou et al. [27] (37.5%) and Takeshima et al. (23%) [28]. On the other hand, mucinous and endometrioid type OC are associated with the lowest rate of lymph node metastases [29]. Regarding the location of PLN in the pelvic and paraaortic areas, the research of Roger et al. [30] showed that the histological subtypes had no influence on the distribution of positive nodes in patients with EOC.
We observed a significantly increased median survival rate for stage II OC patients who had lymphadenectomy when compared with stage II OC patients who had no lymphadenectomy. However, although the retrospective character of our study as well as the surgeon’s independent and subjective decision to perform lymphadenectomy may be the source of bias, our observation supports the current guidelines indicating the need for lymphadenectomy in early stage (I and II) OC. Another limitation of our study is the number of participants; although we included over six hundred women, the patients were analyzed according to the stage of the disease and residual diseases; therefore, several subgroups included a small number of patients.








