Introduction
A sedentary lifestyle and the growth of inappropriate behaviors including ethanol drinking have developed in today’s societies. Alcohol consumption is known as one of the top causes of death by increasing the chance of chronic diseases [1]. Ethanol is also associated with increased hepatic damage, lipid peroxidation, atherosclerosis, and metabolic syndromes, especially dyslipidemia [2, 3]. The most notable impact of ethanol, especially binge drinking, is the elevation of free radicals and the development of oxidative stress [2, 3]. Over time, ethanol-induced damage may be improved; however, finding ways to enhance the recovery and improve the hepatic damage faster is necessary. Some of these methods include regular physical activity and the use of herbal anti-inflammatory compounds.
Studies have shown that performing regular exercise leads to positive effects on lipid profile including elevated high-density lipoprotein (HDL-C) [4, 5]. Elevation of HDL-C, in turn, results in increased antioxidant properties and prevents LDL-C oxidation [4, 5]. The antioxidant and anti-inflammatory properties of HDL-C are associated with paraoxonase-1 (PON-1) [6]. Previous findings have also indicated that physical activity has a positive effect on PON-1 [7-9]. As PON-1 is produced in the liver, ethanol drinking decreases the PON-1 gene expression due to hepatic damage, which is a precursor for the incidence of hepatic diseases, diabetes, obesity, atherosclerosis, increased lipid peroxidation, and dyslipidemia [7-10].
The anti-inflammatory compounds present in herbal medicines are also effective in removing the complications of ethanol consumption. One of these plants is turmeric, with its active ingredient curcumin, which is especially effective in mitigating hepatic damage, improving lipid profile, and enhancing PON-1 gene expression; but the most important role of curcumin is in oxidative stress [11, 12]. It should be noted that curcumin inhibits the nuclear factor kappa B (NF-kβ) signal pathways and enhances oxidant defense, thus leading to reduced hepatic damage from ethanol consumption [13, 14].
The existing studies have not specifically dealt with the investigation of the interactive effect of specific exercises such as swimming. There is also a gap in studying the prescription of curcumin within a shorter period for measuring hepatic damage and changes in PON-1 gene expression following binge ethanol drinking. The non-invasive methods are also very valuable. Therefore, the aim of this study is to investigate the interactive effect of swim training (SWT) and curcumin consumption on PON-1 and NF-kβ gene expression following binge ethanol drinking in male Wistar rats. It is aimed to examine the effect of the combination of these two agents on the length of the treatment period and the durability of the effects following ethanol elimination.
Material and methods
Permissions
The whole experimental protocol in this study was approved by the ethics committee of Islamic Azad University, Central Tehran Branch (No. 10121404942042) and was done in accordance with the NIH (National Institutes of Health) guide for the care and use of laboratory animals (No. 80-23), which emphasized the use of minimal animals being sacrificed and minimal pain imposed during the study.
Animals
Fifty-six male Wistar rats (200-250 g) which were capable of swimming were randomly selected and equally divided into seven groups: dextrose-control (Dext-Con), ethanol-control (Eth-Con), ethanol-saline (Eth-sal), ethanol-dimethyl sulfoxide (DMSO) (Eth-DMSO), ethanol-curcumin (Eth-Cur), ethanol-swimming training (Eth-SWT) and ethanol-SWT + curcumin (Eth-SWT + Cur) (Table 1). To evaluate the effect of curcumin, the results were compared with the group injected with normal saline. In addition, the DMSO group was used to evaluate the effect of DMSO alone (curcumin dissolved in DMSO). The animals were kept in a cage with constant temperature (21 ±2°C), humidity (40-60%), and 12/12 light-dark cycle (light was on at 7:00 a.m.).
Table 1
Timeline of investigation (24 days)*
Binge ethanol induction
The binge ethanol drinking protocol was induced by intragastric gavage every 8 h for 4 days (12 doses in total) in accordance with previous methods [15]. The animals were gavaged with an ethanol diet (25% ethanol w/v in vanilla Ensure; Abbot Laboratories, Columbus, OH) or an isocaloric control diet (dextrose in vanilla Ensure). This initial dose for each animal was 5 g/kg. The subsequent doses were administered depending on the behavior of ethanol up to a maximum of 7.4 g/kg. To ensure the isocaloric control diet, rations were cut for all groups in the binge ethanol drinking protocol. After binge ethanol drinking was induced, the diet changed to the standard and the animals were kept for 6 days without any intervention.
Curcumin preparation
Curcumin (Sigma, St. Louis, Mo, USA) was mixed with 10 mg/ml curcumin solvent (dimethyl sulfoxide: DMSO, Sigma, ST. Louis, MO, USA). The dissolved curcumin (50 mg/kg) was injected intraperitoneally for 2 weeks (five times per week, the same as swimming sessions).
Swimming training
The swimming training was performed 5 times per week for 2 weeks (at 11:00 a.m.). The training was performed in a large water tank (with a diameter of 150 cm) at 32 ±1°C, filled to a depth of 50 cm. Each session of the SWT program began with 20 min and it was increased to an hour (until exhaustion) from beginning to end of interventions. Since the rats are more active at night, but exercises were done during the days, it was finally decided to perform the swimming in a dark room. After each SWT session, the animals were dried with a towel and returned to their cages.
Biochemical assays
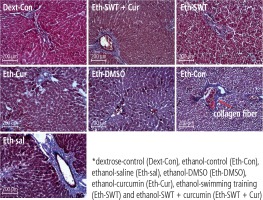
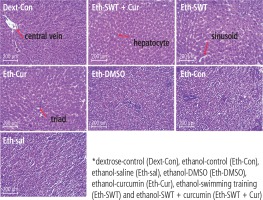
To sacrifice the animals, they were anesthetized with a mixture of ketamine and xylazine (100 and 10 mg/kg, respectively). To investigate gene expression, all samples were taken from the liver (100 mg) and then frozen with nitrogen. Eventually the tissues were stored at –80°C. For histopathological investigations, the liver tissue was kept in formalin 10%. All histological studies were conducted by the hematoxylin-eosin (H&E) method and mason trichrome method for fibrosis assessment, and then the imaging and evaluating were conducted by Image J software.
To perform RNA extraction, a special kit was used (Qiagen, Hilden, Germany: QIAzol Lysis Reagent). RNA concentration was measured (Eppendorf, Germany) with a desired purification of 260 to 280 between 1.8 and 2. Creation of cDNA was also performed using a special kit (QuantiTect Reverse Transcription kit: Qiagen). The created cDNA was kept at –20°C to be used for real-time PCR reactions. To measure gene expression, the realtime PCR quantitative method was applied, using Primix SYBR Green II (Applied Biosystems, Step One, USA). The reactions and their combined experiments (final volume of 20 μl) (consisting of 1 μl of cDNA, 1 μl of forward primer, 1 μl of reverse primer, 7 μl of DEPC water, and 10 μl of SYBR Green II) were conducted twice (Table 2). Further, GAPDH was employed as the reference gene. The thermal program used in the real-time PCR consisted of 95 for 10 min, 60 for 15 s, and 72 for 1 min (replication of 40 cycles). A melting curve was plotted to investigate the accuracy of the data while a standard diagram was drawn to optimize the experimental conditions. Finally, gene expression of data was calculated using Pfaffl’s (2001) method through gene expression in relation with the reference gene [16].
Table 2
Specific primers used in real-time PCR
Statistical analysis
The Kolmogorov-Smirnov test was used to determine the normality of the distribution. All results were expressed as mean ± standard deviation. In order to analyze the data and to investigate the inconsistencies of the observation amongst different groups, the two-way ANOVA method was used, which was then followed by the LSD post-hoc test. Further, the Pearson correlation test was used to determine the potential relationships between the measured variables. Biochemical data were analyzed using SPSS (PASW) 18 (Inc., Chicago, Illinois, USA) and charts were plotted using Origin 6.1 software.
Results
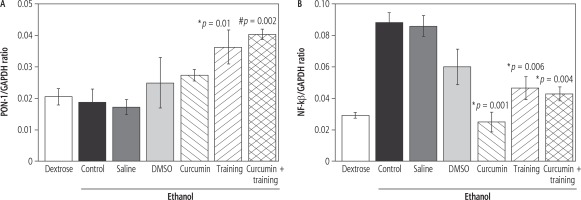
The SWT resulted in enhanced PON-1 gene expression (p = 0.002, F = 11.083) (Fig. 1A). However, curcumin had no significant effect on PON-1 gene expression (p = 0.4, F = 0.899). The post-hoc test indicated that PON-1 gene expression was significantly higher in the Eth-SWT (p = 0.01) and Eth-SWT + Cur (p = 0.002) groups, compared with the Eth-Con group. Furthermore, PON-1 gene expression was significantly higher in the Eth-SWT + Cur group than the Eth-Cur group (p = 0.05) (Fig. 1). SWT did not result in NF-kβ gene expression (p = 0.28, F = 1.187), but curcumin led to a significant decrease in NF-kβ gene expression (p = 0.003, F = 5.333). Interaction of SWT and curcumin also resulted in a significant synergic decrease in NF-kβ gene expression (p = 0.004, F = 9.544). NF-kβ gene expression was significantly higher in the Eth-Con group than the Eth-Cur (p = 0.001) and Eth-SWT (p = 0.006) groups (Fig. 1B).
Fig. 1
Changes in liver PON-1 (A) and NF-kβ (B). *,# Represents significant difference in ethanol-training as well as ethanol-training-curcumin groups compared with ethanol group
Based on the mason trichrome results, the collagen sedimentation rate in the tissues of the ethanol group was significantly higher than in the dextrose group (Fig. 2). In addition, the H&E results showed that hepatocyte death and high cell spaces were more observed in ethanol groups compared with the Dext-Con group. This effect was reduced in the training and curcumin groups. Hepatocytes have vacuolic histoplasts and are seen in some cells without membranes (Fig. 3).
Discussion
The present study indicated that SWT and curcumin resulted in increased PON-1 gene expression and reduced of NF-kβ gene expression. The variables studied significantly improved in the Eth-SWT group compared with the other groups. Exercise is inherently an oxidative stress factor [9, 17, 18]; therefore, the degree of improvement in the Eth-SWT group was lower than that in the Eth-SWT + Cur group. It is possible that with a longer course of SWT, more improvements should be observed. However, all Eth-SWT, Eth-Cur, and Eth-SWT + Cur groups recorded a significant increase in PON-1 as well as other variables, when compared with the control groups. This can be explained by the fact that curcumin and SWT had a significant synergic effect on PON-1. A subset of the oxidative stress generated by exercise may be inhibited through curcumin. That is probably why the PON-1 improved more in the Eth-SWT + Cur group than in the Eth-SWT group. In addition, lack of a significant clinical change in the saline and DMSO groups is another observation suggesting the effectiveness of SWT and curcumin. It is reported that ethanol in rats results in a 25% reduction in PON-1 activity and a 51% reduction in PON-1 gene expression [19]. Although some studies have reported elevated PON-1 and HDL-C at moderate ethanol levels [19], there is no conclusive evidence suggesting alcohol consumption could be controlled through the development of a sense of physiological and psychological demands [20, 21]. Additionally, changes in the concentration of anti-inflammatory agents are different from their function [22].
It is likely that the above effects along with protective effects of curcumin resulted in improving liver function. Likewise, synthesis of HDL-C and PON-1 in the liver also increased. Note that in this study, the interactive or independent effect of curcumin and SWT on PON-1 and reduction of NF-kβ gene expression was mainly observed. Curcumin resulted in the inhibition of inflammatory cytokines of tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), and oxidative stress in the liver and pancreas of rats and mice. However, it had no effect on improving the tissue pathology [11, 12, 23]. In contrast, it has been reported that curcumin also led to inhibition of oxidative stress and reduction of the NF-kβ inflammatory pathway and improvement in the liver tissue [13, 14, 24]. In the present study, observation of some tissue damage in the Eth-Con group can confirm protective effects of curcumin and SWT in other groups.
Factors that have a role in the synthesis of PON-1 are influenced by IL-6. The decrease of PON-1 gene expression under inflammatory conditions can be explained by the fact that after one bout of rigorous exercise elevated IL-6 and decreased PON-1 were both observed [25]. The findings of this study indicate that one course of SWT and curcumin interventions elevates PON-1 and inhibits NF-kβ gene expression. This may be due to adaptation to the exercise routine, the therapeutic affectability of curcumin, and the diminished complications of the quitting period. Nevertheless, statistical findings confirm the effectiveness of SWT and curcumin, as the control groups did not show any significant clinical changes.
The most important point regarding the relationship between rehabilitation training and increased PON-1 is the adaptation of HDL-C in response to regular exercise. This is because PON-1 is attached to HDL-C. The elevation of HDL-C enhances the probability of increased PON-1. On the other hand, adopting a regular exercise routine leads to the increase in protective and antioxidant factors. In the course of one bout of rigorous exercise, free radicals increase. This increase, through the adaptation through signaling pathways, results in improved antioxidant mechanisms [26]. This finding agrees with the existing research indicating that adults who perform regular exercise have significantly higher PON-1 gene expression [7]. Several factors, such as different types of PON and different types of Apo-proteins, have an effect on PON gene expression. However, since PON-1 is activated in the presence of calcium [27], muscle contractions could affect the activity of this enzyme. In addition, in this study, it was observed that curcumin accelerated the adaptation, as the training period was short.
People who regularly exercise exhibit less oxidative stress in response to one bout of rigorous exercise, which is evident from their higher PON-1 function [7-9]. Briefly, regular exercise improved antioxidant defense and inhibited low-density lipoprotein (LDL-C) peroxidation. It is worth noting that the antioxidant activity increases in response to regular exercise. In addition, people with better physical training are more likely to recover from the oxidative stress resulting from rigorous exercise [8, 9]. For example, it has been shown that aerobic training in obese people results in elevated PON-1 and increased leptin and adiponectin [7, 22, 28]. In addition, after six months of aerobic training in obese women, in spite of weight loss, no significant change was observed in HDL-C and PON-1 levels. At the same time, the women’s functional indices of HDL-C and PON-1 were improved [29]. Another reason for the lack of consistency in the results is use of animal and human species under different conditions. Whether people or animals had been physically trained plays an important role in the results. The present research is very different in terms of the utilized methods, and this complicates congruence or incongruence of the results with other studies.
Regular exercise resulted in improved response of PON-1 activity immediately after one rigorous exercise bout, which declined after 2 h and returned to initial levels after 24 h [9]. These findings confirm that oxidative stress resulting from rigorous exercise causes enhanced LDL-C peroxidation and probably PON-1 activity, which in turn inhibits lipid oxidation [17]. This is because rigorous exercise provides oxidative stress and lipid peroxidation conditions [9, 17]. One bout of rigorous exercise results in increased antioxidant agents against increased LDL-C peroxidation [18, 25]. It is noteworthy that PON-1 prevents LDL-C oxidation, as described previously.
Limitations
One of the limitations of this study was the use of a short period of interventions. It was intended to reduce the pain of animals due to the methodology of the study. Also, it was meant to reduce the number of sacrifices, one of the reasons for choosing a small sample size, which can affect the findings of the study. Otherwise, measuring other liver parameters could provide more clear findings. In addition, it has to be remembered that due to the precise control of the experimental procedure, it is difficult to generalize and compare findings with other studies, especially studies on human models.
Conclusions
The findings of this research suggest that the combination of curcumin and SWT may be used to improve the inflammatory state after binge ethanol drinking. It can be concluded that SWT and curcumin may result in enhanced PON-1 as well as reduced NF-kβ gene expression. Therefore, it is suggested that the course of complications in the alcohol quitting period should be shortened, while a combination of curcumin and training is used. Swimming exercises are an appropriate way to rehabilitate via increase floating in the water and weight tolerance, as shown in the present study. In a research study on humans, the intensity of similar exercises should be controlled and documented.