Introduction
Chronic postsurgical pain (CPSP) is defined as pain that persists for at least 3 months after surgery and severely impairs patients’ long-term daily activities and quality of life. The incidence of chronic pain varies between 3% and 85% depending on the type of the surgical procedure, and is estimated as 14.3 and 17.4% after 3 and 36 months after thoracic surgeries, respectively [1]. The development of CPSP is a complex process involving biological, psychosocial, and environmental mechanisms. Intraoperative nerve injury has been suggested as a crucial factor in the emergence of chronic pain due to central sensitization as these repetitive nociceptive stimuli are thought to cause neuropathic pain [2, 3]. The risk factors for chronic postoperative pain include intraoperative nerve injury, duration of surgery, pre-existing pain, young age, female gender, genetic predisposition, psychological vulnerability, and severity of acute postoperative pain. According to the available literature, approximately 40% of patients who develop an acute pain syndrome after thoracotomy subsequently develop a post-thoracotomy pain syndrome (PTPS) [4]. Although video-assisted thoracoscopic surgery technique (VATS) can reduce the incidence of postoperative neuropathic pain regardless of the duration of surgery, chronic pain is still observed after VATS in 20–-25% of patients [5]. To date, several strategies have been used to minimize the progression from acute to chronic pain, promoting the use of multimodal analgesic approaches, including regional analgesia methods [6, 7].
Aim
Our study aimed to evaluate the effects of different regional analgesia methods including thoracic epidural (TE) and fascial plane blocks (serratus anterior plane block, rhomboid intercostal block, thoracic paravertebral block) on the incidence of chronic pain after thoracic surgery.
Material and methods
The medical records of patients who underwent thoracic surgery between January 2016 and June 2020 were reviewed in this retrospective study, following the approval of the local ethics committee (ethics committee approval number: 2020/01-20).
Patients aged 18–65 years who underwent thoracic surgery with ASA (American Society of Anesthesiologists) Scores I–III, successful block (at least 4 dermatomal levels) and available postoperative 6th month follow-up results, who received the same regional anesthesia protocols and agreed to participate in the study were included. The exclusion criteria were as follows: undergoing secondary surgery, uncontrolled diabetes mellitus, mental retardation, antidepressant use, metabolic disorders, and patients using more than one analgesia method.
Patients were called for a follow-up to access the data. Follow-up chart information and patient questionnaires were used for analysis.
The study included 489 patients who met the inclusion criteria and underwent thoracotomy (n = 240) and VATS (n = 249). The analgesics were administered with the patient’s consent, leaving them free to choose.
All patients included in the study were administered premedication with intravenous midazolam at 0.01–0.02 mg/kg and standard monitoring (electrocardiogram, non-invasive blood pressure, and peripheral oxygen saturation). After induction with fentanyl (1–2 µg/kg), propofol (2–3 mg/kg), and rocuronium (0.6 mg/kg), the patient was intubated with a double lumen tube. Anesthesia was maintained with sevoflurane in a fresh gas mixture of 50% air + 50% O2 at 2 l/min. Regional anesthetic methods and intravenous treatments were administered according to clinical protocols. Regional anesthesia protocols were administered by the same anesthesiologist (KO) with the standard dose of 0.3 ml/kg (0.125% bupivacaine) for serratus anterior, and rhomboid intercostal blocks and 15–20 ml (0.125% bupivacaine) for paravertebral block. Thoracic epidural analgesia was obtained with the administration of 6–10 ml of bolus saline solution containing 0.125% bupivacaine (1 mg/ml), followed by its continuous infusion at a rate of 5–8 ml/h (0.1 ml/kg).
Patient-controlled anesthesia (PCA): For the IV PCA protocol, saline solution prepared with 5 mg tramadol per ml was mounted on the PCA device. The PCA device was set to a lock time of 30 min, a demand dose of 25 mg and a daily maximum dose of 400 mg.
Outcome measures:
Primary measures: VAS score (6th month postoperatively)
Secondary measures: Leeds Assessment of Neuropathic Symptoms and Sign (LANSS) pain scale (6th month postoperatively), analgesic medication, postoperative time and analgesia method administered.
The LANSS score is used to differentiate neuropathic pain from nociceptive pain [8].
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) Windows version 23.0 software (SPSS for Windows Inc., Chicago, USA) was used for data analysis. In addition to descriptive statistical methods (frequency, percentage, mean, standard deviation, median, min.–max.), the χ2 test was used to compare qualitative data. The conformity of the data to normal distribution was evaluated by the Kolmogorov-Smirnov and Shapiro-Wilk tests. In cases where the data were not normally distributed, the Mann Whitney U and Kruskal-Wallis test was used for comparisons between affected and unaffected parties. Values with a probability (p) value < 0.05 were considered significant. The Pearson correlation test was used to evaluate correlation.
Results
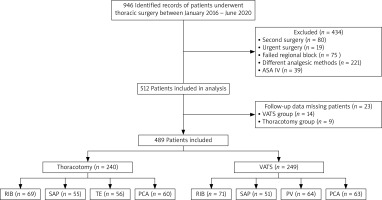
Within the scope of the study, 946 patients were evaluated, of which 512 patients fulfilling the inclusion criteria were included (Figure 1).
Since the follow-up data of 14 patients in the thoracotomy group and 9 patients in the VATS group were missing, the study was completed with a total of 489 patients, 240 in the thoracotomy and 249 in the VATS groups.
In the thoracotomy group, statistical analysis was performed on 69 patients who received rhomboid intercostal block (RIB), 55 who received serratus anterior plane (SAP), 56 who received thoracic epidural anesthesia (TE), and 60 patients who received patient-controlled anesthesia (PCA). The demographic data were similar between the groups (Table I). The RIB group had the lowest VAS score at the 6th month follow-up. In the intergroup comparison, there was a significant difference between the RIB and SAP groups and the TE and PCA groups (Table II).
Table I
Demographic, operative, and LANSS scores of patients undergoing thoracotomy (n = 240)
Table II
VAS and LANSS scores of thoracotomy and VATS patients
The incidence of neuropathic pain (LANSS > 12) after thoracotomy was 37%. The PCA group had the highest LANSS score at the 6-month follow-up (Table I). Intergroup comparison revealed a significant difference between the PCA and RIB, TE and SAP groups (Table II).
The use of analgesics at 6 months after thoracotomy was highest in the PCA group. Statistically significant lower values were found in the RIB and SAP groups (Table III).
Table III
Rates of analgesic use in VATS and thoracotomy patients
| Variable | Drug prescribed n (%) | NSAID n (%) | Paracetamol n (%) | Gabapentin n (%) | Pregabalin n (%) | Tramadol n (%) | Tramadol + paracetamol n (%) |
|---|---|---|---|---|---|---|---|
| Thoracotomy: | |||||||
| RIB (n = 69) | 19 (27) | 10 (14) | 1 (0.4) | 2 (2.8) | 3 (4.3) | 1 (1.4) | 2 (2.8) |
| SAP (n = 55) | 19 (37) | 9 (16.3) | 2 (3.6) | 2 (3.6) | 1 (1.8) | 2 (3.6) | 3 (5.4) |
| PCA (n = 60) | 35 (54.3) | 10 (16.6) | 2 (3.3) | 4 (6.6) | 4 (6.6) | 5 (8.3) | 10 (16.6) |
| TE (n = 56) | 22 (34.2) | 11 (19.6) | 1 1.7) | 1 (1.7) | 1 (1.7) | 5 (8.9) | 3 (5.3) |
| P-value | 0.000 | # | # | # | # | 0.000 | 0.000 |
| Difference | RIB, SAP and TE between PCA | N/A | N/A | N/A | N/A | RIB, SAP between TE and PCA | PCA between SAP, TE and RIB |
| VATS: | |||||||
| RIB (n = 71) | 14 (19.7) | 7 (9.8) | – | 2 (2.8) | 2 (2.8) | 1 (1.4) | 2 (2.8) |
| SAP (n = 51) | 21 (41) | 9 (17.6) | 2 (3.9) | 2 (3.9) | 3 (5.8) | 2 (3.9) | 3 (5.8) |
| PVB (n = 63) | 20 (31.2) | 10 (15.6) | – | 3 (4.6) | 3 (4.6) | 3 (4.6) | 1 (1.5) |
| PCA (n = 64) | 30 (47.6) | 8 (15.8) | 2 (3.1) | 4 (6.2) | 5 (7.9) | 4 (6.3) | 7 (11.1) |
| P-value | 0.000 | # | # | # | # | # | 0.000 |
| Difference | RIB, between SAP, PCA and TE | N/A | N/A | N/A | N/A | N/A | PCA between SAP, TE and RIB |
Statistical analysis was performed on 71 patients who underwent RIB, 51 patients who underwent SAP, 64 patients who underwent paravertebral block and 63 patients who underwent PCA in the VATS group. The groups were similar in terms of demographic data (Table IV). Among them, the SAP group had the lowest VAS score at the 6 month follow-up. There was no statistically significant difference between the VAS scores of the SAP, PV, and RIB groups. The VAS scores of the PCA group were higher than the VAS scores of the other three groups and were found to be statistically significant (Table IV).
Table IV
Demographic, operative, and LANSS scores of patients undergoing VATS (n = 249)
The incidence of neuropathic pain (LANSS > 12) after VATS was 18%. The PCA group had the highest LANSS score at the 6-month follow-up. Comparison between groups showed a significant difference between the PCA and PV groups and the RIB and SAP groups. Higher LANSS scores were found in the PCA and PV groups (Table IV).
The use of analgesics 6 months after VATS was highest in the PCA group. Statistically lower values were found when compared between the RIB and the other three groups (Table III).
Age and gender were not correlated with the chronicity of pain at 6 months after thoracotomy (p = 0.767, r = –0.019) or VATS (age: p = 0.150, r = –0.092) The relationship between pain scale scores and gender was analyzed by χ2 test. While VAS scores were unrelated to gender (thoracotomy; p = 0.440, VATS; p = 0.105), LANSS scores were related (thoracotomy; p = 0.001, VATS; p = 0.105). Duration of surgery and chronic pain scores in the 6th month were the thoracotomy and in the VATS group no correlation was detected (thoracotomy; LANSS: p = 0.435, r = –0.051, VAS: p = 0.364, r = –0.059, VATS; LANSS: p = 0.345, r = –0.060, VAS: p = 0.561, r = –0.037).
Discussion
In this study investigating the effect of different regional analgesia (RA) methods on chronic pain after thoracic surgery, patients were divided into two groups who underwent thoracotomy or VATS. The incidence of chronic pain after thoracotomy and VATS was 37% and 18%, respectively. The regional anesthesia modalities used for postoperative analgesia in patients undergoing thoracotomy were RIB, SAP, TE and PCA. VAS scores were lower in the RIB and SAP groups than in the TE and PCA groups. While LANSS scores did not differ between regional anesthesia techniques, higher scores were found only in patients who underwent PCA. The regional anesthesia techniques used after VATS were RIB, SAP, PVB and PCA. VAS and LANSS scores were lower in the SAP and RIB groups. The analgesic use rate was higher in the PCA group in patients who underwent both thoracotomy and VATS.
A PTPS is a serious complication of thoracic surgery that lasts for months or years and is accompanied by a series of painful sensations [9, 10]. This chronic pain can lead to disruption of daily life and long-term disability [11, 12]. Pain after thoracotomy is associated with costal retraction, trauma to intercostal nerves, visceral damage causing visceral pain, and placement of chest drains [13]. Although most surgical injuries occur around peripheral nerves that activate nociceptive pain, the inflammatory and neuropathic changes caused by surgery have the potential to cause changes in both the peripheral and central nervous system and contribute to the development of chronic postoperative pain. One of the most important aspects in preventing the chronicity of pain is adequate postoperative analgesia.
Regional anesthesia techniques such as thoracic epidural analgesia (TEA), paravertebral block (PVB), Erector Spinae, SAP and RIB blocks can be used for postoperative analgesia after thoracic surgery, in addition to the intravenous route [14]. How RA affects these factors is still not clear, but proposed mechanisms include suppression or blockade of nociceptive nerve impulses, regulation of signals from glial cells, and minimization of synaptic plasticity of neurons. In their meta-analysis, Weinstein et al. reported that the use of intraoperative regional anesthesia in addition to general anesthesia reduced the risk of PTPS [15]. Epidural analgesia and paravertebral block have been shown to reduce the risk of chronic pain after some types of surgery by blocking nociceptive input to the spinal cord [16].
Although different area blocks are used for post-operative pain, TEA is still the gold standard method for analgesia after thoracic surgery. The literature on PTPS includes studies mostly on the efficacy of the TEA method. Previous studies have reported the effectiveness of TEA in preventing PTPS [17]. Kampe et al. compared thoracic epidural analgesia and opioid-based postoperative analgesia and found statistically significant lower NRS (numeric rating scale) scores in the TEA group at 6 months postoperatively. In the group that developed postoperative chronic pain, higher pain scores were found in the first 5 days postoperatively and the necessity of a highly effective postoperative pain treatment was emphasized to prevent the development of chronic pain after thoracotomy [18].
There are studies investigating the effect of different regional anesthesia techniques on PTPS. Patients who underwent TEA, PVB and intercostal block-in were evaluated with VAS at the first and sixth months and a 23% decrease of PTPS incidence was found in patients who underwent TEA [19]. In their study to investigate chronic pain after thoracotomy with TEA or PVB, there was no statistical difference in the 6-month values of NRS and S-LANSS scores between patients undergoing TEA and PVB; however, it was noted that patients who developed PTPS had significantly reduced quality of life and the addition of a neuropathic component was a further problem [20]. More recently, area blocks have been described for postoperative analgesia after thoracic surgery [21]. There are limited data on the effect of these regional techniques on PTPS. Semyonov et al. found no statistically significant decrease in PTPS after a SAPB for postoperative pain control compared to the standard group but reported a decrease in the percentage of PTPS incidence when compared to the standard group [22]. In our study, VAS scores measured 6 months postoperatively in thoracotomy patients were lower in patients who underwent RIB and SAP block than in patients who underwent regional anesthesia. Regional anesthesia modalities achieved inferior chronic pain relief results compared to the intravenous route.
The number of surgeons preferring less invasive VATS to open thoracotomy increased in the recent years [23]. VATS reportedly can reduce the incidence of acute postoperative pain and postoperative neuropathic pain, regardless of the duration of surgery. However, chronic pain after VATS is still observed in 20–25% of patients [12]. It was reported that moderate to severe pain episodes were more frequent 52 weeks after surgery after anterolateral thoracotomy compared to VATS [23]. In another study, the incidence of chronic pain related to thoracic surgery was 27% at 6 months and similar between thoracotomy and VATS patients. In this study, the incidences of chronic pain at 3 and 6 months after thoracotomy were 47% and 33%, respectively, while the incidences of chronic pain at 3 and 6 months after VATS were 29% and 25%, respectively [12].
The incidence of chronic pain after VATS, as well as the factors affecting its occurrence, were investigated in different studies. In the study reporting a 35.3% incidence of CPSP after VATS, female gender, age and acute pain were identified as risk factors [24]. In another study, factors such as age, gender, BMI, duration of surgery and anesthesia methods were not associated with the occurrence of CPSP after VATS [20]. The common result of both studies is that acute pain after surgery is an independent risk factor for the occurrence of CPSP [20, 24]. Bayman et al. found that patients with more severe pain in the first 3 days after surgery were more likely to develop chronic chest pain at 6 months [12]. Therefore, postoperative acute pain management is as important after VATS as after thoracotomy. PVB is also frequently used in thoracic surgery analgesia where TEA is considered the gold standard. Nowadays, fascial plane blocks such as SAP and RIB have been added to these methods. Wang et al. investigated the efficacy of 3 different regional anesthesia methods for acute pain after VATS. They found no differences between the CPSP incidences of groups that received PVB and SAP blocks, a group that used only PCA and a group that received TEA [20]. In another study including patients who underwent TEA, PVB and intercostal nerve blockade, all three methods reduced the incidence and severity of CPSP. However, the most significant effect was found in the TEA group [25]. Literature data are insufficient on the effect of field blocks on CPSP after VATS. When VAS score values were analyzed in this study, the lowest VAS score was found in the PVB block group at 6-month follow-up. It can be said that regional anesthesia methods are more effective when compared with patients who received PCA. On the other hand, simple evaluations such as NRS and VAS score reportedly inadequately evaluate CPSP and are not objective in the evaluation of chronic pain in patients undergoing thoracic surgery as it may be confusing in the detection of pain originating from other regions and due to other causes in patients who underwent surgery for cancer and similar conditions. Chronic pain measurement tests may lead to more objective results. LANSS scores and the incidence of CPSP were lower in patients undergoing thoracotomy and VATS with regional anesthesia methods.
Limitation: The retrospective nature of this study is the first limitation. We could not include many potential predictors of CPSP. Although factors such as pre-existing pain status and psychological state, which are controversial predictors of CPSP, can be excluded, the lack of detailed information on postoperative pain scores may be considered the second limitation.
Conclusions
In this study evaluating the incidence of CPSP after thoracotomy and VATS procedures after thoracic surgery, LANSS and VAS scores defining neuropathic pain were lower in both groups of patients receiving regional anesthesia. Similar to pain scores, the rate of analgesic use was lower in the regional anesthesia groups. This study concluded that regional anesthesia techniques reduce chronic pain.