Introduction
Colorectal malignancies occur frequently and are the fourth most common cause of malignancy-related mortality in the world. Approximately 1,096,000 new cases of colon cancer are estimated to be diagnosed in 2018, while approximately 704,000 new cases of rectal cancer are expected worldwide. In addition, rectal cancer is the 10th most deadly, with 310,000 deaths, representing 3.2% of all cancer deaths worldwide [1]. During colorectal surgery, iatrogenic injuries to the urogenital system are well-known problems. In one large series, the risk of ureteral injury in colorectal surgery was reported as 0.24–5% [2, 3]. The risk factors for ureteral injury are malignancy, history of previous surgery, radiotherapy (RT) history, inflammatory bowel disease, diverticulitis, and massive intraabdominal bleeding. Abdominopelvic resection and sigmoidectomy in colorectal cancer surgery are the most common surgical procedures for ureteral injury. Moreover, the distal 1/3 of the ureter is the most frequent location of injury. Unfortunately, the anatomical changes after surgery and fibrotic adhesions increase the organ laceration risk in recurrent cases and secondary operations [3, 4].
The local recurrences in rectal cancer have decreased significantly with the standard use of neoadjuvant chemoradiotherapy (CRT) and total mesorectal excision (TME). Despite this, the local rectal cancer recurrence rate after curative surgery is 4–11%. Performing re-resections in suitable patients who develop isolated local recurrence has been suggested because of an increase in the survival [5, 6]. Most of the recurrences develop within the first two years; therefore, the current guidelines suggest biannual tomographic control during the first three years [7]. Anatomical anomalies are the main reasons for ureteral injuries during surgery. However, the use of computed tomography urography (CTU) has been increasing over the last two decades, and the use of this imaging method has made important contributions to the detection of urinary tract pathologies and anatomical variations [4, 8, 9].
There are no previous investigations in the literature evaluating the effects of rectal cancer surgery on the anatomical localisation of the ureters. Therefore, the aim of this study was to investigate the changes in the anatomical localisations of the ureters via CTU in patients undergoing rectal cancer surgery.
Material and methods
This study was registered at clinicaltrial.gov (NCT03007667) after receiving approval from the local Ethics Committee. Verbal and written informed consent was obtained from the participants. The study period was between 1 November 2016 and 30 September 2017.
Inclusion and exclusion criteria
The study group consisted of patients ≥ 18 years old, with adenocarcinoma located in the rectum. The inclusion criteria were as follows:
American Society of Anaesthesiologists (ASA) score of I–III,
normal renal function,
no history of major abdominal surgery,
no RT history in the pelvic region,
rectal carcinoma suitable for curative surgery (those graded as T4 according to pelvic magnetic resonance imaging [MRI] were excluded),
sphincter protective surgery with an open surgical procedure,
surgical resection with a TME,
no intraoperative ureteral injuries,
no urogenital system congenital anomalies,
no urogenital system surgical interventions,
no anatomical deviations, such as scoliosis or fractures in the bony elements of the vertebrae or pelvis,
no inflammatory bowel disease or diverticulitis,
no allergies to contrast media,
sufficient filling of contrast media in both of the ureters up to the bladder.
CTU evaluation protocol
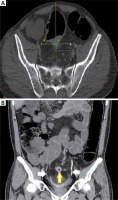
The patients diagnosed with rectal cancer were evaluated via tomography to determine the presence of metastases, operability, and staging. When needed, a pelvic MRI and endorectal ultrasound (ERUS) were performed to evaluate the local disease. The CT scans were conducted in the radiology department of our hospital under the supervision of a radiology specialist (BAÖ). The CTU was performed in the standard supine position with 64-slice, 1-mm sections using an Aquilion CT scanner (Toshiba Corporation, Otawara, Japan). Before imaging, a 1.5 ml/kg dose of iodised contrast media was infused at a rate of 3 ml/min, following the intake of 1.5 l of water 2 h previously. After the contrast medium infusion, images were taken of the arterial phase at the 30th second, portal venous phase at the 70th second, late venous phase at the 120th second, and excretory phase limited to the pelvic region at between the 8th and 12th minute [8, 9]. Two anatomical lines were determined to measure the anatomical changes in the ureters. The first of these was a vertical line reaching the midline of the spinous processes of the vertebrae. The second line, sacral 1 and sacral vertebra 2, was identified as the transverse line passing through the middle of the joint. The distances from the junction of the midvertical line with the transverse line to the ureters were measured. The right ureter distances to the midvertical line measured preoperatively and postoperatively were defined as R1 and R2, respectively. The left ureter distances to the junction measured preoperatively and postoperatively were defined as L1 and L2, respectively (Fig. 1).
Fig. 1
A) The tomographic axial planar measurement of the right and left ureters. B) The white arrows show the ureter in the coronal tomography section, while the yellow arrow shows the tumour
The tumour localisation in the rectum was determined according to the distance to the dentate line as follows: 1–5 cm was lower, 6–10 cm was mid, and 11–15 cm was upper. All of the surgical procedures were done by the same team using an open technique and a standard TME. The anastomosis was performed using a circular stapler, and a drain was placed in the pelvic region. The duration between the two CT sessions was determined as the time between the preoperative CT scan and the one performed at the first CT control. The demographic properties, body mass index (BMI, kg/m2), ASA score, and comorbid conditions of each of the patients were recorded. In addition, the application of neoadjuvant RT and/or postoperative chemotherapy was determined. Any postoperative complications that developed before or after 30 days following the procedure were defined as early and late complications, respectively. The pathological evaluations in this study were performed using the 7th version of the American Joint Committee on Cancer’s tumour, node, metastasis (TNM) staging system [10].
Statistical analysis
The Statistical Package for the Social Sciences version 22 (SPSS Inc., Chicago, IL, USA) was utilised for the biostatistical analysis. The patient data was stated as the standard deviation, minimum-maximum value, and percentage in the required fields. The Shapiro-Wilk’s test was use for the numerical variables, and the significance of the changes in the anatomical localisations of the ureters were evaluated using a paired samples t test. A p value less than 0.05 was considered to be significant.
Results
Curative surgery was performed in 41 patients with rectal cancer in our clinic between 1 November 2016 and 30 September 2017. Twenty-three patients were excluded and 18 were included in this study. The mean age of the patients was 58.9 ±10.4 years (range: 37–82 years), and the male-to-female ratio was 1/1. The comorbidity rates of the patients were as follows: hypertension 44.4% (n = 8); diabetes mellitus, 22.2% (n = 4); coronary artery disease, 11.1% (n = 2); and chronic obstructive pulmonary disease, 11.1% (n = 2). The mean BMI value was 28.3 ±3.19 kg/m2. The ASA score rates were 16.6% (n = 3) for ASA I, 44.4% (n = 8) for ASA II, and 38.9% (n = 7) for ASA III. Ten (55.5 %) of the patients were given neoadjuvant RT. The mean duration between the two CT sessions was 7.7 ±1.5 months (range: 6–12 months). The demographic and clinical data of the patients are summarised in Table 1.
Table 1
Patient characteristics
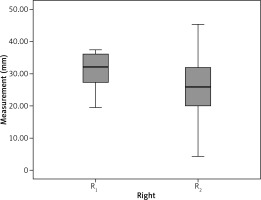
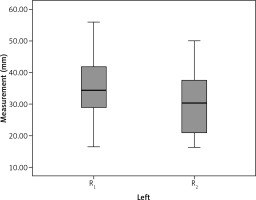
The mean preoperative distance between the right ureter and the midvertebral line (R1) was 30.9 ±5.4 mm, and the mean distance between the left ureter and the midvertebral line (L1) was 34.5 ±9.9 mm. The postoperative distances of the right and left ureters (R2 and L2, respectively) were 26.4 ±9.1 mm and 29.5 ±9.9 mm, respectively. The postoperative measurements showed that 83.3% (15/18) of the right ureters shifted medially, while 16.7% (3/18) of them shifted laterally. The postoperative measurements of the left ureters showed that 88.8% (16/18) of them shifted medially and 11.2% (2/18) of them shifted laterally (Table 2). The differences between the two measurements on the right and left were 4.5 ±92 mm and 4.9 ±4.6 mm, respectively. The anatomical change amount in the left ureters was statistically significant (p ≤ 0.001). Figures 2 and 3 show the position changes of the ureters. During the early postoperative period, leakage developed in two patients whose right ureters shifted laterally. During the late postoperative period, one patient developed a stricture on the anastomosis line, and another developed a vaginal fistula from the anastomosis line. There were no complications in the third patient, whose right ureter shifted laterally.
Table 2
Results of preoperative and postoperative measurements
Both patients whose left ureters deviated laterally also had lateral changes in their right ureters. Whereas there were neither early nor late complications in one of these patients, the other developed leakage and a stenosis in the late postoperative period. The patient who had a lateral deviation of the right ureter, but a medial deviation of the left ureter developed vaginal fistula.
The early postoperative complications consisted of anastomosis leaks in two patients and urinary retention, a wound infection, candidemia, ileus, and anastomosis bleeding in one patient each. All of these complications were treated medically and conservatively. Mortality did not develop in this study population.
Discussion
Studies investigating the effects of rectal carcinoma surgery with a TME on the anatomical localisation of the pelvic ureters are limited. In our study, the CTU scans showed that the ureters moved closer to the midline in most of the patients. There were medial deviations in 15/18 (83.3%) right ureters and 16/18 (88.8%) left ureters, whereas a medial change in the position was frequent and more distant in the left ureters than in the right ones. Because the study population was limited, it is difficult to determine a valid reason for this difference. The inclusion of the sigmoid mesocolon during the TME procedure and the surgical dissection through the trace of the left ureter may have been the causes. One of the interesting results of this study was the presence of the same positional change in two of the three patients with lateral ureter deviations. The other patient with a lateral deviation of the right ureter developed a fistula from the anastomosis line to the posterolateral wall of the vagina during the second postoperative month. The medial change in the position of the left ureter was 11.8 mm in this patient, and the distance was significantly greater than the mean value. The inflammation due to the fistula may have contributed to the right deviation in the anatomical localisation. Although the dislocation in the right ureter was not statistically significant (p = 0.052), the dislocation in the left ureter was significant (p ≤ 0.001).
Injuries to the urogenital system in colorectal surgery are likely complications because of the close anatomical vicinity. The ureter is the most frequently injured organ, and the complexity of the surgery increases the risk of damage. During colorectal surgery, the risk of injuring the ureter increases in certain circumstances, such as severe inflammation due to diverticulitis, inflammatory bowel disease, and pelvic infections. A locally advanced tumour stage, rectal carcinoma recurrence, and previous RT also increase the anatomical distortions [11, 12]. Ninety per cent of all iatrogenic injuries to the ureter develop in the distal part of the ureter [13]. A curative resection is possible via the development of new surgical techniques and procedures. However, fibrosis, scar tissue, and the loss of dissection planes due to previous surgery and RT increase the risk of injuring a neighbouring organ. The insertion of stents into the ureters is advised, if pelvic exenteration is not planned. Although it is under discussion, it is suggested that rate of ureteral injury is increased in laparoscopic colorectal surgery [2, 3, 14–17].
The use of CTU has been increasing over the last two decades, and it has made important contributions to the diagnosis of urinary tract pathologies and the detection of anatomical variations. CTU is the first-line diagnostic procedure in the evaluation of microscopic haematuria, and the excretory phase is the ideal phase to image the ureter and bladder [8, 9]. Moreover, sufficient distention in the distal parts of the ureters and bladder with contrast media filling is important to define the traces of the ureters. Therefore, it is recommended that the images be taken 8–12 min after the contrast media application to provide a suitable excretory phase. Attempts have been made to reduce the radiation dose by giving the contrast media in split boluses [18]. Four patients were excluded from this study because they were not provided with enough contrast media to fill the distal ureter.
This study did have some limitations. For example, it included a small study population, which complicated the definition of the factors influencing the dislocation of the ureters. In addition, the changes in the anatomical localisations were measured in two dimensions, while the changes in the anterior and posterior directions were not evaluated. Finally, a comparison related to the effects of the surgical procedures, such as abdominoperineal resection, low anterior resection, and open and laparoscopic techniques, could not be done.
Conclusions
Rectal carcinoma curative surgery including a TME may cause the medial dislocation of the ureters. Further larger case-control studies are necessary to define the factors causing these changes in anatomical localisations. CTU may be useful to prevent injury if surgery is to be performed for local recurrence. Surgeons should take into account the fact that the ureters move closer to the midline in patients with rectal carcinoma surgical histories when considering surgical treatments in recurrent cases.