Introduction
Excimer laser coronary angioplasty (ELCA) was developed with the aim of improving the outcome of conventional balloon angioplasty [1]; therefore, its original target was limited to a very specific subset of lesions, such as saphenous vein graft, total occlusions, calcified lesions, ostial lesions, lesions greater than 20 mm in length, and balloon dilation failures [2–4]. However, an excimer laser, which can vaporize thrombus, suppress platelet aggregation, and ablate the underlying plaque, has recently been shown to be effective in patients with acute coronary syndrome (ACS) [5]. Although rare, vessel perforation is the most terrible complication of ELCA. Early studies regarding the complications of ELCA in its introductory era revealed that patients with vessel perforation were mainly treated via coronary artery bypass grafting (CABG) [6]; however, the necessity of CABG is decreasing as a result of technological advances, as well as improvements in intervention technique. Therefore, the prognosis of patients who were successfully treated for vessel perforation via ELCA without CABG is not fully addressed. Also, the risk factors related to ELCA-induced vessel perforation in the era of a new indication, ACS, and a new generation excimer laser catheter are unknown.
Aim
The purpose of this study was to investigate the current prognosis and mechanism of vessel perforation as induced by ELCA.
Material and methods
We retrospectively analyzed patients who underwent ELCA at Ogaki Municipal Hospital between February 2016 and December 2018. This study was approved by the research review board of Ogaki Municipal Hospital and conducted according to the Helsinki Declaration. Because of its retrospective nature, written informed consent from the participants was waived; however, we excluded the patients who refused to participate in the study.
A pulse-wave xenon chloride excimer laser (Spectranetics CVX-300, Spectranetics, Colorado Springs, Colorado) was applied. It had a 308 nm wavelength, a pulse duration of 135 ns, and an output of 165 mJ/pulse. The laser catheters consisted either of concentric tips (sizes of 0.9, 1.4, 1.7, and 2.0 mm; Vitesse C, Spectranetics) or eccentric tips (sizes 1.7 and 2.0 mm; Vitesse E, Spectranetics). Initial energy parameters for lasing were set at a fluence of 45 mJ/mm2 and 25 Hz and increased if ablation resistance was encountered. The type and size of the laser catheter were chosen by the operators according to the target lesion morphology and degree of stenosis. To facilitate laser-transmitted pressure waves, the saline flush technique was applied in all cases. Retrograde lasing and the degree of debulking were left to the discretion of each operator. Perforation was defined as persistent extravascular collection of contrast medium beyond the vessel wall with or without associated clinical complications [6].
Results
During the study period, we performed ELCA for 399 patients, with 415 lesions. Patient and procedural characteristics are summarized in the Table I. The mean age of the patients was 69.0 (standard deviation = 11.2) years, 40% were diabetic, while 24% had a prior history of percutaneous coronary intervention (PCI). Approximately 85% of the lesions were associated with ACS. Half of these lesions were located in the left anterior descending artery. Of these, coronary perforation was identified in eight patients (1.9%). The patient and procedural characteristics of these eight patients are summarized in Table II. The perforation site for each patient is shown in Figure 1 A1–7. A clear image was unavailable for patient no. 4.
Table I
Patient’s characteristics
[i] Values are the mean ± standard deviation (SD), n (%), or median (interquartile range) as appropriate. PCI – percutaneous coronary intervention, CABG – coronary artery bypass graft, eGFR – estimated glomerular filtration rate, STEMI – ST-elevation myocardial infarction, NSTE-ACS – non-ST-elevation acute coronary syndrome, MI – myocardial infarction, AP – angina pectoris, RCA – right coronary artery, LMCA – left main coronary artery, LAD – left anterior descending artery, LCX – left circumflex artery.
Table II
Patients’ clinical and procedural characteristics
| Patient no. | Age/sex | Target lesion | Indication | ELCA size | Eccentric type | Perforation site | Treatment (acute) | Treatment (chronic) | Pseudo-aneurysm | Cause |
|---|---|---|---|---|---|---|---|---|---|---|
| 1* | 57/M | LAD (diagonal) | STEMI | 1.7 | – | Carina, distal | Balloon | Coil embolization | + | Over size, bifurcation, bending |
| 2 | 50/M | RCA | STEMI | 1.4 | – | Carina, distal | Balloon | CABG | + | Contrast, bifurcation |
| 3 | 72/M | LAD | NSTE-ACS | 1.7 | + | Carina, distal | Balloon | Observation | + | Eccentric plaque, bifurcation |
| 4 | 77/M | RCA | sAP | 0.9 | – | Outer side | Covered-stent | – | NA | CTO, calcified lesion |
| 5 | 71/F | LAD | STEMI | 1.7 | – | Outer side, proximal | Balloon | DES | + | Bifurcation, bending |
| 6* | 75/M | LCX | NSTE-ACS | 1.4 | – | Carina, distal | Covered-stent | – | + | Bifurcation, bending |
| 7 | 65/F | LAD | NSTE-ACS | 1.7 | + | Carina, distal | Covered-stent | – | + | Eccentric plaque, bifurcation, bending |
| 8 | 81/F | LAD | STEMI | 0.9 | – | Carina, distal | Covered-stent | – | + | Bifurcation, bending |
M – male, F – female, LAD – left anterior descending artery, RCA – right coronary artery, LCX – left circumflex artery, STEMI – ST-elevation myocardial infarction, NSTE-ACS – non-ST-elevation acute coronary syndrome, sAP – stable angina pectoris, CABG – coronary artery bypass graft, DES – drug-eluting stent, CTO – chronic total occlusion.
Figure 1
The location of perforation and pseudoaneurysm formation. A1–7 – The location of perforation and pseudoaneurysm formation in patients no. 1–3 and 5–8. B – The tip of the laser catheter would face the relatively thin portion of the plaque and underlying vessel wall. Therefore, the vessel wall may be perforated as a result of laser energy ablating tissue in a radial, rather than in an axial direction in the vessel lumen
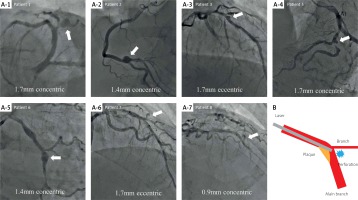
The location of perforation occurred at the carina posterior to the bifurcation in six lesions (excluding patients no. 4 and 5), and a pseudoaneurysm formation was seen in seven cases (excluding patient no. 4). The perforation may be attributed to the bifurcation in seven lesions, an eccentric plaque as revealed by intravascular ultrasound in two lesions, incomplete saline flush (remaining contrast) in one lesion, and chronic total occlusion in one lesion. For secure bleeding control, a covered stent (Graftmaster, Abbott Vascular, Santa Clara, California) was implanted at the time of index PCI in three patients (patients no. 6–8). Pseudoaneurysm formation was seen even at the time of index PCI in these 3 patients.
Discussion
In this study, we have shown that most ELCA-induced vessel perforation occurred at the side of the carina posterior to the bifurcation, and that most perforations led to pseudoaneurysmal changes in several weeks following the index PCI, even in cases where temporal hemostasis was medically achieved.
This study highlighted the risk factors related to ELCA-induced vessel perforation and needs of early angiographic follow-up. Recently, advances in PCI technique and the progressive aging of patients with several comorbidities resulted in the enlarged indication of PCI and the subsequent increase of lesion complexity treated by PCI. To obtain satisfactory results for those complex lesions, the indication of adjunctive use of ELCA combined with drug-eluting stent was gradually spreading. However, the reduction of complications, especially vessel perforation, is mandatory before it can be used more widely. The likelihood of vessel perforation in this study was 10 times higher compared with that in the nation-wide PCI registry [7], though the incidence was similar to that in other recent ELCA registries [8, 9]. Hence, our findings have important clinical implications, serving to help interventional cardiologists better recognize lesions susceptible to perforation and thus lead to reduction and appropriate management of ELCA-induced vessel perforation.
The presumed reasons that the bifurcation of the trachea (the carina) was susceptible to perforation include: 1) non-discriminative tissue ablation of ELCA compared with differential cutting of rotational atherectomy; 2) the stiffness of the laser catheter, which prevents it from smoothly tracking the curvature of the vessel [10]; 3) tissue ablation without physical contact; and 4) the nature of plaque, which rarely affects the carina (Figure 1 B). Despite the rare description of pseudoaneurysm formation after ELCA [6, 11], the likelihood of pseudoaneurysm formation could be presumably higher, as in the present study, because the range of laser-induced arterial wall dissolution could be larger than that of balloon-induced arterial tear [12].








