Summary
Carotid intima-media thickness (CIMT) can be regarded as a surrogate marker for cardiovascular disease. A possible relationship between CIMT above 0.8 mm and obesity (BMI > 30 kg/m2) was noted. Long-term statin therapy may indicate the relationship between CIMT and rosuvastatin (at least 20 mg/day) or atorvastatin (at least 40 mg/day) administration. Long-term statin therapy is associated with a reduced likelihood of having CIMT above 0.8 mm, although the presented results are statin-type and dosage-dependent.
Introduction
Morbidity related to cardiovascular disease (CVD) is a leading epidemiological problem [1]. As atherosclerosis affects many arterial beds, the carotid artery performance assessment gives a unique opportunity through ultrasound examination to establish atherosclerotic disease, even by intima-media complex estimation. The correlation between abnormal carotid intima-media thickness (CIMT) in asymptomatic patients and increased risk for cardiovascular events has been noted in epidemiological studies [2]. CIMT is defined as the distance between the lumen-intima and media-adventitia and represents the thickness of two wall layers [3]. It can be regarded as a surrogate marker for cardiovascular disease. Though increased CIMT is reported in one-fourth of the adult population [4], its prognostic value for multisite arterial disease ranges is reported [5]. CIMT can be regarded as an indicator of vascular bed alterations by multiple factors affecting the arterial walls.
In a multicenter study by Fu et al. [6], age, male sex, arterial hypertension, diabetes, dyslipidemia and geographic distribution were found to be related to CIMT. There is a growing interest in the potential influence of environmental factors on cardiovascular morbidity [7, 8].
Lipid-lowering agents such as statins have proven to reduce future risk and regression of atherosclerotic plaques [9]. The therapy is considered an exceptionally important element in the primary and secondary prevention of cardiovascular events [10]. Its beneficial effect in patients with established carotid atherosclerosis was noted and related to reduction of cerebrovascular events [11].
Hydroxymethylglutaryl-CoA reductase possesses pleiotropic actions beyond lowering cholesterol, including anti-inflammatory effects and immune function alteration [12]. The relation between decrease of neutrophil extracellular traps, a novel marker of innate immunity activation, and high-dose statin therapy has been recently postulated [13]. As CIMT is regarded as an early marker of subclinical atherosclerosis in high-risk patients, the high-dose statin therapy in dyslipidemic patients improved lipid profile and showed beneficial effects on CIMT progression in previous studies [14].
Previous studies not only indicated an association between CIMT and heart failure (HF), but also suggested that CIMT can be regarded as a possible marker of HF progression [15–17]. In previous research [18], the interplay between CIMT progression and heart failure with preserved ejection fraction (HFpEF) was related to decreased arterial distensibility.
The definition of HFpEF is defined by clinical symptoms provoked by exertion and objective evidence of laboratory results and diastolic dysfunction followed by elevated left ventricular filling pressures conducted at rest, fulfilling the criteria of echocardiographic algorithms [19]. As there is growing frequency of HFpEF diagnosis, the proper therapeutic approach is essential from an epidemiological perspective.
Aim
The aim of the study was to relate long-term high-dose statin therapy to CIMT, a possible marker of HF progression, in a retrospective analysis of patients presenting with HFpEF.
Material and methods
There were 77 (47 female and 30 male) consecutive patients with a median age of 69 (62–75) years admitted to the Hypertension and Internal Medicine Department presenting with preserved ejection fraction heart failure symptoms in NYHA class 2.0 (0.5) for clinical evaluation in 2024. Laboratory tests, echocardiography, carotid ultrasound, and cine angiography were performed. The analysis did not include patients presenting with acute syndromes, reduced left ventricular ejection fraction, or previous cardiovascular interventions.
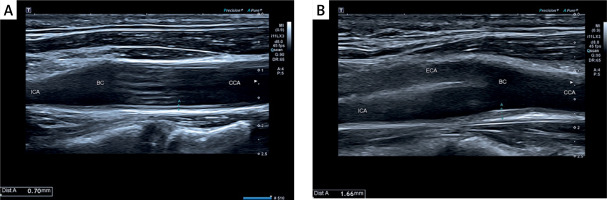
Among diagnostic procedures, the carotid and vertebral arteries’ morphology combined with intima-media complex characteristics was determined by an experienced ultrasonographer on the Philips EPIQ CVx system using a high-frequency broadband linear probe (3.0–12.0 MHz). CIMT measurements were performed on the posterior wall of the carotid artery 10–20 mm distally from the common carotid bulb.
The intima-media complex was routinely examined in ultrasound imaging, and patients were divided according to the CIMT 0.8 mm cut-off value, as presented in Figures 1 A, B.
Based on carotid ultrasound results and CIMT, patients were divided into two groups, as presented in Table I.
Table I
Demographic and clinical characteristics of studied groups with long-term pharmacotherapy
| Parameters | Whole analyzed group (n = 77) | CIMT > 0.8 mm Group 1 (n = 15) | CIMT < 0.8 mm Group 2 (n = 62) | P-value Group 1 vs. 2 |
|---|---|---|---|---|
| Demographic: | ||||
| Age [years] median (Q1–Q3) | 69 (62–75) | 73 (69–77) | 69 (61–74) | 0.06 |
| Sex (M/F), n (%) | 30 (39)/47 (61) | 5 (33)/10 (67) | 25 (40)/37 (60) | 0.63 |
| BMI [kg/m2] median (Q1–Q3) | 27.8 (24.8–31.7) | 29.8 (26.4–31.4) | 27.6 (26.4–31.4) | 0.32 |
| BMI > 30, n (%) | 27 (35) | 7 (47) | 20 (32) | 0.32 |
| Clinical, n (%) | ||||
| Dyslipidemia | 72 (94) | 15 (100) | 57 (92) | 0.58 |
| Arterial hypertension | 69 (90) | 13 (87) | 56 (90) | 0.69 |
| Diabetes mellitus | 15 (20) | 3 (20) | 12 (19) | 0.61 |
| Active smoking | 1 (27) | 3 (20) | 18 (29) | 0.49 |
| Long-term pharmacotherapy, n (%): | ||||
| β-blockers | 61 (79) | 12 (80) | 49 (79) | 0.94 |
| ACE-I | 35 (46) | 6 (40) | 29 (47) | 0.64 |
| ARB | 18 (23) | 3 (20) | 15 (24) | 0.74 |
| CCB | 32 (42) | 4 (27) | 28 (45) | 0.20 |
| SGLT2 | 15 (20) | 3 (20) | 12 (19) | 0.96 |
| ASA | 62 (81) | 11 (73) | 51 (82) | 0.44 |
| Ezetimibe | 27 (35) | 4 (27) | 23 (37) | 0.46 |
| Statins | 70 (91) | 12 (80) | 57 (92) | 0.18 |
| Atorvastatin | 19 (25) | 2 (13) | 17 (27) | 0.26 |
| Atorvastatin ≥ 40 mg/day | 14 (18) | 1 (7) | 13 (21) | 0.20 |
| Rosuvastatin | 51 (66) | 10 (67) | 41 (66) | 0.98 |
| Rosuvastatin ≥ 20 mg/day | 31 (40) | 4 (27) | 27 (44) | 0.24 |
| High daily statin dose* | 45 (58) | 5 (33) | 40 (65) | 0.041 |
Statistical analysis
The normality of the distribution of the variables was tested using the Shapiro-Wilk test. The t-test, Cochran-Cox test, Mann-Whitney test, or Fisher’s exact test was used where applicable to compare the variables between the two groups. Logistic regression was performed to analyze the laboratory data which predicted the CIMT above 0.8 mm. We reported odds ratios (ORs) with 95% confidence intervals (CIs). A receiver operator characteristic (ROC) analysis was carried out. A statistical analysis was performed using JASP statistical software, Version 0.13.1 (JASP Team (2020)). A p-value < 0.05 was considered statistically significant.
Results
There were 15 (5 male and 10 female) patients composing group 1 who were characterized by CIMT diameter above 0.8 mm. Neither laboratory, echocardiographic, nor cine angiographic results differentiated the groups (Table II). The pharmacotherapy included in the analysis was continued unchanged for 22 (6–36) months. The median time of statin therapy in groups 1 and 2 was 30 (18–54) vs. 18 (6–42) months (p = 0.120), respectively.
Table II
Laboratory, ultrasound, echocardiographic, and cine angiographic results
[i] ALT – alanine transaminase, CIMT – carotid intima-media thickness, Hb – hemoglobin, Hb1Ac – glycated hemoglobin, HDL – high-density lipoprotein, HDL/LDL – high- to low-density lipoprotein ratio, Hct – hematocrit, LICA – left internal carotid artery, LDL – low-density lipoprotein, LVA – left vertebral artery, LVED – left ventricular end-diastolic diameter, LVEF – left ventricular ejection fraction, LVES – left ventricular end-systolic diameter, n – number, Plt – platelets, RICA – right internal carotid artery, RVA – right vertebral artery, Q – quartile, WBC – white blood count.
The uni- and multivariable model was created for prediction of elevated CIMT above 0.8 mm, including demographic and clinical characteristics and laboratory results, followed by long-term lipid-lowering agent therapies, as presented in Table III. The multivariable model indicated possible relations of CIMT above 0.8 mm with obesity (BMI > 30 kg/m2) (OR = 11.86, 95% CI: 2.5–54.02, p = 0.001) and high-statin therapy (OR = 0.18, 95% CI: 0.04–0.08, p = 0.024).
Table III
Uni- and multivariable models for increased CIMT (above 0.8 mm) prediction
| Parameter | Univariable model | Multivariable model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Demographic | ||||||
| Age > 70 [years] | 1.00 | 0.32–3.08 | 0.99 | |||
| Sex (female) | 0.50 | 0.14–1.76 | 0.28 | |||
| BMI > 30 [kg/m2] | 6.62 | 1.83–23.96 | 0.004 | 11.86 | 2.60–54.02 | 0.001 |
| Clinical | ||||||
| Arterial hypertension | 1.78 | 0.20–15.70 | 0.60 | |||
| Diabetes mellitus | 1.44 | 0.26–7.94 | 0.68 | |||
| Active smoking | 1.44 | 0.43–4.84 | 0.56 | |||
| Laboratory | ||||||
| HDL/LDL (ratio) > 2.52 | 2.14 | 0.55–8.22 | 0.27 | |||
| Lipoprotein a | 1.00 | 0.99–1.02 | 0.66 | |||
| Therapy: | ||||||
| High-dose statin therapy* | 0.36 | 0.11–1.18 | 0.09 | 0.18 | 0.04–0.80 | 0.024 |
| Ezetimibe | 0.617 | 0.18–2.16 | 0.45 | |||
| Any coronary disease | 1.13 | 0.34–3.76 | 0.84 | |||
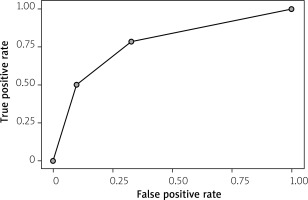
The receiver operator curve (ROC) for CIMT over 0.8 mm predictions composed of two parameters – BMI over 30 and high statin dose (at least 20 mg of rosuvastatin or 40 mg of atorvastatin daily) – was characterized by an AUC of 0.794 with an F-measure of 0.417 and yielding sensitivity of 35.7% and specificity of 91.8%, as presented in Figure 2.
Discussion
Our retrospective analysis identified a possible relation between daily high doses (at least 20 mg of rosuvastatin or 40 mg of atorvastatin) of statin therapy and intima-media complex.
The risk stratification based on risk equations is the cornerstone of CVD prediction. Though it may provide a good estimate at the population level, it may fail to assess the individual’s risk projection. The personalized approach based on easily accessible and non-invasive ultrasound carotid artery examination is regarded as a validated good CVD marker. Carotid intima-media thickness, describing the distance between two layers in the carotid artery wall, is regarded as a subclinical radiologic early marker of atherosclerotic disease. Its measurements were found to be predictive in multiple clinical situations, such as the co-existence of diabetes, arterial hypertension, dyslipidemia, or obesity [20]. The results should be interpreted with other markers by multidisciplinary teams to prevent disease progression. The CIMT may be regarded not as a surrogate of atherosclerosis but as a marker of vascular aging, so multidisciplinary teams should interpret the results with other markers to prevent disease progression [21].
Nevertheless, according to our study, the significance of CIMT for age-related changes in vascular beds or atherosclerosis progression can be modified by statin therapy. As age is a predominant CVS risk factor and free radical-induced damage over the life-span is postulated, the possible CIMT modification is clinically relevant and crucial in the aging population.
Atherosclerosis is regarded as a chronic inflammatory condition orchestrated by cytokines, chemokines, and acute-phase reactants that interact with arterial wall cell populations [22]. The relation between inflammatory markers and carotid disease was noted in previous studies [23–25]. The inflammatory activation is involved in almost every stage of atherosclerotic plaque formation, including endothelial stress dysfunction, followed by monocyte recruitment, low-density lipoprotein (LDL) oxidation, and inner wall thickening [26]. Our results indicate the possible modulatory effect of high-dose statin therapy on CIMT that may be related to the pleiotropic effect of statin on inflammatory activation.
The anti-inflammatory role of rosuvastatin in endothelial nitric oxide synthase expression upregulation and inflammatory apoptosis prevention has been noted in previous animal studies [27]. Rosuvastatin can stabilize or reverse atherosclerotic plaques by protecting the vascular endothelium against inflammation, including lectin-like oxidized low-density lipoprotein receptor 1, a transmembrane glycoprotein involved in foam cell formation [28]. Its anti-inflammatory effect was noted in chronic allergic asthma in animal models [29]. In the JUPITER trial [30], high-dose statin therapy upregulated bioactive lipids’ anti-inflammatory and antioxidant properties, supporting their pleiotropic effects. In acute coronary syndromes, the rosuvastatin could persistently down-regulate glycoproteins of the Dickkopf family (DKK) released from the platelets and endothelial cells that modulate wingless (Wnt) signaling pathways critical for atherosclerosis [31].
Atorvastatin’s protective effect on the vascular endothelium is widely recognized. In animal models, it counteracted angiotensin II-induced vascular endothelial injury by ubiquitinating ATP5A (ATP synthase mitochondrial F1 complex subunit alpha) [32]. In a rat model, Chu et al. [33] observed reduced accumulation of vascular smooth muscle cells via phosphorylation inhibition of p38 mitogen-activated protein kinases (p38 MAPKs). In the study by Chen et al. [34], atorvastatin administration significantly reduced homocysteine reduction, and C-reactive protein downregulation combined with carotid atherosclerosis regression was noted in elderly patients. In stroke patients, atorvastatin therapy was associated with a reduction in carotid artery CIMT [35].
Study limitations. The single-center retrospective study was based on single CIMT measurements and related to long-term statin therapy in an elderly group of patients. The real-life study was performed on stable, consecutive patients, limiting the sample size, with an unbalanced representation between the two groups regarding age (p = 0.06) but not co-morbidities.
Recommendations: A prospective multicenter study, including time-related changes in CIMT in relation to type and dosage of statin therapies, is required to confirm the hypothesis presented here.
Conclusions
The results from the retrospective single-measurement analysis on long-term statin therapy may indicate a relation between CIMT and rosuvastatin (at least 20 mg/day) or atorvastatin (at least 40 mg/day) administration. Long-term statin therapy is associated with a reduced likelihood of having CIMT above 0.8 mm, although the present results depend on statin type and dosage. A prospective study is required to confirm these results.