Introduction
Liver transplantation (LT) is the only effective treatment method in patients with end-stage liver disease such as liver cirrhosis and hepatocellular cancer. Due to limited resources, LT cannot be performed on every patient with end-stage liver disease, and many countries have had to create a candidate list for this. It is crucial to have scores that will determine the prognosis of those on the list. The most common score used to determine priority for LT is the model for end-stage liver disease (MELD) score [1]. MELD-sodium (MELDNa) and Child-Turcotte-Pugh (CTP) score are the other scores [2]. MELDNa is a score created by adding sodium to the MELD score, calculated with the patient’s creatinine, international normalized ratio (INR), bilirubin, and Na values. The CTP score is determined by the presence of encephalopathy, presence of ascites, bilirubin, INR, and albumin values.
The MELD score has been confirmed to be a good predictor of pre-LT survival among different populations of patients with advanced liver disease [1]. MELDNa was found to be more helpful in determining survival [3]. However, the effectiveness of MELD and CTP in prognosis after LT for all patients is unclear and should be improved [4, 5]. Few studies provide information about the short-term prognosis of patients undergoing LT [6, 7]. However, determination of mortality after LT is as important as determining the priority of patients waiting for organs in resource use. The high-cost treatment of LT patients admitted to the intensive care unit (ICU) continues here as well. Scores predicting mortality are available for all patients admitted to the ICU. The APACHE score is widely used in the ICU because it can classify the severity of illness and predict hospital mortality. However, the APACHE score is impractical due to the large number of variables to be evaluated for liver disease and transplantation. Therefore, the tendency to research and develop the MELD score after LT and studies in this direction have increased [8, 9].
Despite the many advantages of the MELD score, it cannot accurately predict the survival of approximately 15-20% of patients. It is possible that the addition of variables that are better determinants of liver and renal function may improve the predictive accuracy of the model [1]. It would be prudent to consider an indicator for the effect of liver dysfunction on the hematological system. Currently, there is no hematological parameter that includes platelets in available scores. Platelet disorders are common in patients with liver failure. Portal hypertension plays an essential role in coagulopathy in liver disease, reducing the number of circulating platelets. However, platelet function and thrombopoietin secretion are also impaired in patients with liver disease [10]. We know that the mean platelet volume (MPV) value indicates the activation of platelets, and it is a predictor of mortality in some diseases, according to studies[11, 12]. A few studies have also found evidence that it may indicate inflammation and fibrosis in liver diseases [13, 14]. This evidence suggested that MPV might be a predictor of liver failure. Some studies have evaluated MPV with scores in patients with chronic liver disease, but these are few [15]. MPV negatively correlates with platelet count in severe patients. Therefore MPV/platelet count (MPR) has been proposed as a better indicator of platelet function. In many diseases, increased MPR causes adverse events such as pneumonia after ischemic stroke, sepsis, critical illness, and malignant tumor [16]. For these reasons, we thought that MPR would be more decisive in prognosis in patients who underwent LT and included it in our study.
Research is ongoing to develop scores that will more precisely determine mortality after LT, both before transplantation and at admission to the ICU. In our study, we aimed to investigate the disease-specific score and improve the existing scores to better determine the prognosis of patients after LT. For this purpose, we evaluated the relationship of pre- and post-LT MELDNa and CTP scores with 30-day mortality after LT. We also planned to investigate whether MPR could contribute to improving the score.
Material and methods
Our retrospective study included liver transplant patients at Dokuz Eylul University Hospital, Department of General Surgery in the Hepatopancreaticobiliary Surgery and Liver Transplantation Unit between 2011 and 2019. A total of 178 patients hospitalized in the ICU after transplantation were evaluated. Patients under 18 and whose data could not be reached were excluded from the study.
Demographic, clinical data
Patient-related data were obtained from outpatient medical records and the hospital’s electronic data system. Patients’ age, gender, etiology of liver disease, presence of cancer, donor type (living donor/deceased donor), duration of cold ischemia, length of stay in the ICU, and 30-day mortality were recorded.
Laboratory data and scores
Patients’ blood tests (biochemistry, complete blood count, and coagulation tests) were performed on the day of admission to the hospital’s general surgery service and at the time of admission to the ICU after LT. Patients’ abdominal magnetic resonance and ultrasound imaging reports were reviewed, and whether there were masses and/or ascites and the size of the masses were evaluated. In addition, patients’ need for hemodialysis and the presence of encephalopathy were examined from the medical records. The presence and degree of encephalopathy were obtained from the results of the gastroenterology consultation and recorded. Since the number of patients with hyperglycemia was very low, sodium values were accepted without correction for hyperglycemia. The MELDNa and CTP scores of the patients at hospitalization were calculated from the obtained medical data. The MELDNa score at the time of admission to the ICU after LT was calculated, too. Since the CTP score included the criteria for ascites and encephalopathy and there would be no change in these criteria, it was not recalculated after LT. The CTP score was calculated with an online score calculator called MDcalc Child-Pugh score [17]. The MELDNa score was calculated with an online score calculator called MDcalc MELDNa. In this calculator, MELD = 10 × (0.957 × ln [creatinine]) + (0.378 × ln [bilirubin]) + (1.12 ln [INR])) + 6.43, MELDNa = MELD score – Na – 0.025 × MELD × (140 – Na) + 140 as calculated. Sodium was limited in a range of 125-140 mmol/l, and if outside of these bounds, it was set to the nearest limit [18]. We preferred this score over the sodium integrated MELD score, which was determined for patients over 12 years of age. We thought that the MELDNa score was more suitable for developing the score, which is the aim of our study.
MELD exceptions criteria were taken into account in the calculations. MELDNa scoring was calculated using a different method (Québec MELD HCC exception point system) for patients with hepatocellular carcinoma (HCC) [19].
Surgical procedure and transfusion strategy
The same surgical team performed liver transplant procedures involving deceased or living donors, and standard techniques were used. The transfusion strategy in all patients in the peroperative period was as follows:
If hemoglobin (Hb) is below 7 g/dl, erythrocyte suspension transfusion was performed.
If the platelet count was below 50 × 103/µl, platelet suspension transfusion was performed [20].
Intensive care
The anesthesiologist and intensive care specialist followed the patients together admitted to the ICU after LT according to the predetermined follow-up protocol.
After a liver transplant, calcineurin inhibitors (cyclosporine/tacrolimus), corticosteroids, and mycophenolate mofetil were used as a standard initial immunosuppressive treatment for all patients. Mammalian targets of rapamycin inhibitors (sirolimus, everolimus) were used immediately after the development of renal toxicity due to calcineurin inhibitor use. The patients whose general condition stabilized after extubation were transferred to the general surgery service.
Statistics
Statistical analyses were performed using SPSS software version 24.0. Categorical variables were presented as counts and proportions (%), and quantitative variables were represented as mean ± standard deviation (SD) or median (interquartile range). Normal distribution tests were used to evaluate whether the quantitative variables conformed to the normal distribution. The normal distribution was analyzed according to groups with and without mortality within 30 days. When the number of individuals in these groups was less than 50, it was evaluated with the Shapiro-Wilk test.
Patients with and without mortality within 30 days were compared with the t-test, χ2, and Mann-Whitney U test in terms of descriptive features.
MELDNa, CTP score, MPV, MPR values were compared in patients with and without mortality within 30 days using the Mann-Whitney U test and t-test. PostLT-MELDNa score performed in the ICU was evaluated with ROC analysis in terms of mortality predictive value.
Logistic regression analysis was performed to determine the predictive factors for 30-day mortality after LT. Factors with p < 0.05 in univariate analysis were analyzed separately using multivariate analysis. The odds ratio and 95% confidence intervals were calculated for each factor. A model was created with multivariate analysis.
Results
Our study included 178 (135 male, 43 female) patients. The mean age of the patients was 50.53 ±12.66 years. In our study group, dominant causes for LT were hepatitis B virus cirrhosis (34.3%). Fifty-two of all our patients have HCC. One hundred three transplants were performed from living donors. Mortality occurred within 30 days in 25 of 178 patients (Table 1).
Table 1
Clinical characteristics of patients and comparison of survivors and nonsurvivors
As a result of the evaluation of the patients in terms of scores: preLT-MELDNa (at admission to surgery service) median value was 19, postLT-MELDNa (at admission to ICU) median value was 18. PostLT-MELDNa was statistically significantly higher in patients with mortality within 30 days (p < 0.05). The length of stay in the ICU was higher in patients with mortality within 30 days (Table 2).
Table 2
Comparison of scores and pre-post liver transplantation values
Since MELD assessment was based on malignancy severity in patients with HCC, MELDNa and MPR were re-analyzed without HCC patients to exclude the effect of malignancy. PostLT-MELDNa was significant in predicting 30-day mortality also in patients without HCC (p < 0.05) (Table 3).
Table 3
Comparison of survivor and nonsurvivor patients without hepatocellular carcinoma
Age, postLT-MELDNa, and CTP score were significant in predicting 30-day mortality when evaluated with univariate analysis (p < 0.05). Mortality rate increased with increasing age and score values. Since MPV and MPR were not significant in univariate analysis, the multivariate analysis could not be performed together with the score. Multivariate analysis was performed with independent factors obtained in univariate analysis. A model was created as a result of the multivariate analysis. In this model, age and postLT-MELDNa were found to be more effective in their associations in terms of predicting mortality (Table 4).
Table 4
Univariate and multivariate analysis, model for predictors of 30-day mortality
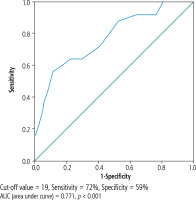
PostLT-MELDNa score was significantly higher in patients who died. In this respect, the cutoff value of the score was examined by ROC analysis, and it was found that the value of 19 (72% sensitivity, 59% specificity) was a good determinant threshold (Fig. 1).
Discussion
The most important result of our study was to investigate and improve a specific score for the prognosis after LT patients; the postLT-MELDNa score was an independent factor in predicting 30-day mortality. In addition, we found that the pre-LT CTP score was also significant. As a result of the multivariate analysis of our patients, we found a model in which the postLT-MELDNa score was more effective in predicting 30-day mortality with age. MPR, on the other hand, did not contribute to the improvement of the score, as it was not decisive for mortality.
In our study, the preLT-MELDNa score was not predictive of 30-day mortality after LT. In a similar study conducted before, MELD and MELDNa scores were weak predictors of postoperative graft failure and mortality [21]. Since there was a need for a scoring system, the studies continued following this study, carried out in 2011. A review considered 37 of these studies; in 15 of the studies, there was no relationship between preLT-MELD and post-transplant survival, while 22 of them were found to be associated, but their predictive rate was low; therefore, weak evidence was accepted [22].
The persistence of uncertainty for the PreLT-MELDNa score seems to have brought re-evaluation in the ICU to the fore. The APACHE II score has been used for prognosis in the ICU for a long time. However, the APACHE II score has been considered to have limitations since there is no liver transplant diagnostic category, and it overestimates in-hospital mortality in post-LT patients [23]. It was thought that it would be more appropriate to use disease-specific scores in ICU. Therefore, the APACHE IV score was developed, with 116 detailed admission diagnosis options, including postoperative LT. Although another study found the APACHE IV score superior to the MELD score in terms of ICU survival, this study had a limitation of LT patient number and recommended further study [24]. In addition, the APACHE IV score is likely to create difficulties in standardization due to the large number of variables to be evaluated. We evaluated the postLT-MELDNa score in patients admitted to the ICU after LT, as it is disease-specific and easily applicable. We found it significant in predicting 30-day mortality. A similar study found that the postLT-MELD score measured at admission to the ICU determines mortality in patients who underwent LT from a living donor [25].
Since it is more practical to use the MELDNa score in patients admitted to the ICU after LT, we think this score will benefit more in predicting mortality. The fact that postLT-MELDNa was predictive for 30-day mortality in the analysis of our patients without HCC suggests that it has a high value in terms of disease-specific score.
We performed ROC analysis to determine the cutoff at which we can more clearly evaluate the sensitivity and specificity. The postLT-MELDNa score of 19 points predicted 30-day mortality with 72% sensitivity. We could not find a large-scale study evaluating MELDNa in the ICU after LT in the literature. A study including 777 patients with HCV cirrhosis admitted to the ICU for other reasons showed that MELDNa as a disease-specific score had a high predictive value for determining ICU mortality in liver patients and found the cutoff value to be 20 [26].
Our study found that age is one of the independent factors predicting 30-day mortality in patients who underwent LT. The mortality rate increased with increasing age. As a result of multivariate analysis, we obtained a model including postLT-MELDNa and age. We found that postLT-MELDNa was much more effective in predicting 30-day mortality when evaluated with age. Previous studies found the iMELD score a predictor of mortality in patients with chronic liver disease [27]. iMELD = MELD + (0.3 × age) – (0.7 + Na) + 100. The age and postLT-MELDNa model in our study may indicate that iMELD is valuable in predicting prognosis of post-LT patients with chronic liver disease.
We found no significant association for mortality when we investigated pre- and postLTMPR to improve the score. In fact, many studies support that our hypothesis is strong. Ding et al.’s [28] study found that MPR is a predictor for 3-month mortality in patients with HBV cirrhosis. Zampieri et al.’s [29] study found that the increase in MPV in the first 24 hours of admission to the ICU in critically ill patients was associated with increased mortality. Our study has the limitation of being single-centered. In addition, blood product transfusions during the operation could not be evaluated. All of these may have affected the results related to MPR. There are also some limitations regarding MPV. One evaluation determined that MPV studies should be performed with many multicenter, standardized measurements because the difference between abnormal and normal was minimal and affected by ethnicity, age, and gender [30].
Conclusions
It is important to determine the prognosis after LT patients are admitted to the ICU using the disease-specific score. This score may provide more certainty than standard ICU scores. In our study postLT-MELDNa was a predictor of mortality specifically for LT patients. The MELDNa score, which is currently used in the prognosis of candidates awaiting LT, may also be useful for the prognosis of patients after LT in the ICU.
Approval of the Bioethics Committee for the research, approval number: 2020/26-77 (date: 26.10.2020).