Summary
High-sensitivity cardiac troponin T (hs-cTnT) can be detected in patients with heart failure to predict the prognosis. The major finding of our study is that hs-cTnT changes over time with different trends between ischemic heart failure (IHF) and non-ischemic heart failure (NIHF), which could be an important risk factor for the prognosis of patients with IHF and NIHF.
Introduction
Heart failure (HF) is a rapidly growing public health issue with an estimated prevalence of 64.34 million individuals globally [1]. Estimation of prognosis for morbidity, disability and death helps patients, their families and clinicians decide on the appropriate type and timing of therapies and assists with planning of health and social services and resources [2]. Even if the clinical manifestations of the different types of HF are similar, the pathological mechanisms and clinical prognoses of ischemic heart failure (IHF) and non-ischemic heart failure (NIHF) are different. Early identification of precursors of HF is important for the diagnosis and treatment of HF [2–4].
Highly sensitive assays for measurement of cardiac troponin (hs-cTn) have been widely used in the diagnosis of myocardial infarction (MI) [5]. The prognostic role of hs-cTn is not only used for patients with MI but also for patients with HF [6–11]. Myocardial injury, an important pathophysiological mechanism of HF, is persistent when there is elevated hs-cTn in HF [12–14]. High-sensitivity cardiac troponin T (hs-cTnT), above the 99th percentile upper reference limit, is more sensitive and specific than cardiac troponin T in identifying myocardial injury at a much earlier time [5]. And hs-cTnT can be earlier detected than N-terminal pro-B-type natriuretic peptide (NT-proBNP) in minor myocardial injury, showing a more accurate evaluation of prognosis in HF [15, 16].
Aim
At present, there is no definite study on hs-cTnT between IHF and NIHF, and it is still not clear how to use hs-cTnT to predict the prognosis of IHF and NIHF. Therefore, this study focuses on hs-cTnT to evaluate its prognostic role between IHF and NIHF.
Material and methods
Study population
Patients were enrolled from Beijing Tsinghua Changgung Hospital between November 2016 and December 2018. The study was approved by the Beijing Tsinghua Changgung Hospital Research Ethics Committee. The diagnosis of HF was based on typical symptoms and signs according to the 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic HF [2]. The IHF group was made up of patients with obstructive coronary heart diseases whose coronary stenosis was more than 50% evaluated by coronary angiography (CAG) or coronary computed tomography (CCT). The NIHF group was chosen from patients with non-obstructive coronary heart disease, including atrial fibrillation, hypertension, dilated cardiomyopathy, and valvular heart disease, that had been previously definitely diagnosed. The exclusion criteria were acute myocardial infarction, radiofrequency ablation, and/or pacemaker implantation within 1 month, acute pulmonary embolism, chronic kidney disease (estimated glomerular filtration rate (eGFR) < 30 ml/min/173 m2), sepsis, rhabdomyolysis, acute cerebral infarction, and acute cerebral hemorrhage.
Study protocol
According to the primary cause of HF, patients were divided into two groups: an IHF group and a NIHF group. Baseline data including gender, age, body mass index (BMI), blood pressure (BP), heart rate (HR), and other data were recorded. Peripheral blood samples for measuring serum levels of hs-cTnT, NT-proBNP and other indexes were compared between the two groups. Then the ventricular and atrial function and dimensions were evaluated by echocardiography. Patients were telephoned or seen in outpatient clinics at different time points (1, 6, 12 months after discharge), and their endpoint events were re-hospitalization for HF and all-cause death.
Measurement of hs-cTnT
Blood samples for assays of hs-cTnT were taken from peripheral veins at different times when the patients were admitted to the hospital, 2–5 h, 6–24 h, and 24 h–7 days after admission. All the blood samples were stored at room temperature (25°C) and centrifuged at 3000 rpm for 20 min. Hs-cTnT assay was based on electrochemiluminescence technology (Elecsys 2010, cobas e411, Roche Diagnostics). This assay had the lower limit detection of 0.001 ng/ml, coefficient variation of < 10% at a limit of 0.013 ng/ml. In the present study, elevated hs-cTnT was defined as > 0.014 ng/ml.
Statistical analysis
All data were analyzed and processed using SPSS20 statistical software. Continuous and categorical variables were reported as mean ± standard deviation (SD) and number (n) (%) respectively, and tested by the t-test and χ² test between two groups where appropriate. Hs-cTnT and NT-proBNP were reported as median and tested by the Mann-Whitney U test. The levels of hs-cTnT over time and with different New York Heart Association (NYHA) class were analyzed by generalized estimating equations. The rate of re-hospitalization and all-cause death were calculated with the Kaplan-Meier method and the Cox regression method. A receiver operating characteristic (ROC) curve was used to analyze the role of hs-cTnT in predicting the endpoints in HF according to the Youden index.
Results
Baseline characteristics
Baseline characteristics of 65 patients with IHF and 95 patients with NIHF are displayed in Table I. Patients with NIHF had a higher heart rate (HR) and lower complication rate of hyperlipidemia compared to patients with IHF (p < 0.05). Patients with IHF were mostly in NYHA class II–III while patients with NIHF were mainly in NYHA III–IV (p < 0.05). Through echocardiography, patients with NIHF had a larger left atrial dimension (LA) and right ventricular dimension (RV) (p < 0.05).
Table I
Comparison of baseline characteristics between IHF and NIHF
[i] ACEI – angiotensin-converting enzyme inhibitor, ARB – angiotensin receptor blocker, BMI – body mass index, DBP – diastolic blood pressure, eGFR – estimated glomerular filtration rate, HDL-C – high-density lipoprotein cholesterol, HR – heart rate, LDL-C – low-density lipoprotein cholesterol, IVS – interventricular septum, LA – left atrial dimension, LVEDD – left ventricular end diastolic dimension, LVEF – left ventricular ejection fraction, LVESD – left ventricular end systolic dimension, NT-proBNP – N-terminal pro-brain natriuretic peptide, NYHA – New York Heart Association, RV – right ventricular diameter, SBP – systolic blood pressure, TC – total cholesterol.
Variation of hs-cTnT levels
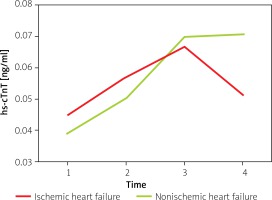
The variation of hs-cTnT levels over time is shown in Figure 1. With the passage of time, hs-cTnT first increased and then decreased in the IHF group, but showed a continuously elevated trend in the NIHF group. The level of hs-cTnT varied statistically significantly over time, but membership of the IHF or NIHF group showed no effect on this level. Patients with different NYHA classes had various levels of baseline hs-cTnT (Table II). It seemed that the higher the NYHA class in patients was, the higher was the baseline hs-cTnT.
Re-hospitalization rate for heart failure and all-cause mortality
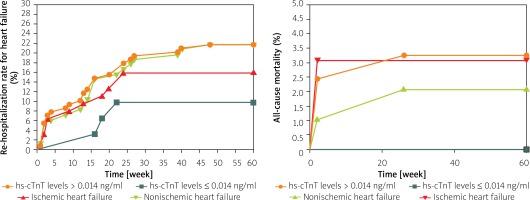
During the follow-up, 28 patients with baseline hs-cTnT levels > 0.014 ng/ml were re-hospitalized for HF and 3 patients with baseline hs-cTnT levels ≤ 0.014 ng/ml; 10 patients were re-hospitalized for HF in the IHF group and 21 patients in the NIHF group. The Kaplan-Meier method showed that patients with a baseline hs-cTnT level > 0.014 ng/ml had a significantly higher re-hospitalization rate compared with those with baseline hs-cTnT levels ≤ 0.014 ng/ml (23.7% vs. 7.0%, p < 0.05) (Figure 2 A). But the re-hospitalization rate for HF was not statistically different between IHF and NIHF groups (Figure 2 A). Moreover, 4 patients with baseline hs-cTnT level > 0.014 ng/ml died of all causes; 2 patients with IHF and 2 patients with NIHF died. The Kaplan-Meier method showed no statistically significant difference between patients with baseline hs-cTnT levels > 0.014 ng/ml and patients with baseline hs-cTnT levels ≤ 0.014 ng/ml, or between IHF and NIHF (Figure 2 B).
Figure 2
A – Re-hospitalization rate for heart failure in different groups. B – All-cause mortality in different groups
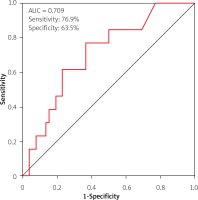
Adjusted for age, NYHA class, NT-proBNP, and LVEF, Cox regression analysis showed that baseline hs-cTnT was independently related to re-hospitalization and all-cause death between IHF and NIHF (p < 0.05). Additionally, the cut-off of hs-cTnT (0.0275 ng/ml) was determined by the receiver operating characteristic (ROC) analysis to predict the composite endpoints in IHF (AUC = 0.709, 95% CI: 0.561–0.856, sensitivity: 76.9%, specificity: 63.5%, p < 0.05) (Figure 3), and the AUC showed no significant difference in NIHF.
Discussion
This study demonstrated that the hs-cTnT levels over time showed an increasing trend in NIHF while first increasing and then decreasing in IHF. Adjusted for age, NYHA class, NT-proBNP, and LVEF, baseline hs-cTnT was independently related to re-hospitalization and all-cause death between IHF and NIHF. Furthermore, the optimal hs-cTnT cut-off of 0.0275 ng/ml was derived to predict the re-hospitalization rate for HF and all-cause death in IHF. Hs-cTnT was found to be an important biomarker to evaluate the re-hospitalization rate for HF and all-cause death in patients with HF.
Whatever the primary disease of HF was, myocardial injury actually existed and affected the functions and outcomes of HF [17–21]. Therefore, myocardial injury resulted in increased hs-cTnT levels in both IHF and NIHF patients. Ischemic myocardial injury could directly cause myocardial cell necrosis, and also could promote the release of growth factors that cause proliferation of the extracellular matrix and remodeling of myocardial tissues [22]. In addition, the direct effects of ischemic factors and the interaction of multiple factors may lead to higher hs-cTnT levels in IHF than those in NIHF. As functional and structural mechanisms can affect the physiological function of myocardial ischemia, the assessments of functional ischemia, including coronary flow reserve (CFR), cardiac magnetic resonance (CMRI), fractional flow reserve (FFR), index of microvascular reserve (IMR), positron emission tomography (PET), and single photon emission computed tomography (SPECT) [23], were essential to patients with HF. It is necessary to perform future studies that can assess the relation between functional ischemia of the myocardium and the dynamics of hs-cTnT in patients with HF.
Val-HeFT and GISSI-HF studies found that the hs-cTnT levels over time present a decreasing trend in HF [24]. But there was lack of classification of etiology with HF patients in testing hs-cTnT over time. In our study, hs-cTnT levels were tested over time between IHF and NIHF, showing first increasing and then decreasing levels in IHF but a continually increasing trend in NIHF. We took samples from peripheral veins for assays of hs-cTnT when the patients were admitted to the hospital, 2–5 h, 6–24 h, and 24 h–7 days. But in those two studies, hs-cTnT was measured at randomization and after 3 months (GISSI-HF) or 4 months of follow-up (Val-HeFT) [24]. First of all, the different test time may contribute to the difference in hs-cTnT levels over time. There were also some reasons for the discrepancy, including the pathological processes of IHF and NIHF, and the treatment strategies for various causes of HF. Time and other factors could act individually and interact to influence hs-cTnT levels. Therefore, a large amount of research data is needed to analyze further the changing trends of hs-cTnT over time in IHF and NIHF.
de Antonio et al. found that the hs-cTnT levels were directly related to the severity of HF [25]. The greater the severity of HF was, the higher were the hs-cTnT levels. In our study, with increasing NYHA class, the hs-cTnT levels displayed an upward trend. With the deterioration of disease, the cardiac systolic and diastolic functions decreased, causing myocardial fibers’ fibrosis and activation of the sympathetic nervous system and renin–angiotensin–aldosterone system. Those changes promoted myocardial remodeling and myocardial injury marked by increased hs-cTnT. Because the degree of myocardial injury was different at the different levels of cardiac function, hs-cTnT levels may also vary with the cause and degree of myocardial injury.
Figald et al. reported that hs-cTnT was associated with re-hospitalization for HF and mortality in HF, which was a predictive indicator of cardiovascular events [6, 26, 27]. Yan et al. reported that adjusted for age, sex, and classical cardiovascular risk factors, hs-cTnI could be an independent factor for the prognosis of HF, and optimal hs-cTnI cutoff values of 0.0026 ng/ml for women and 0.0042 ng/ml for men were derived for selecting individuals at risk [28]. In our study, hs-cTnT was independently related to re-hospitalization rate for HF and all-cause mortality by the Kaplan-Meier method and Cox regression method. And an optimal hs-cTnT cut-off of 0.0275 ng/ml for IHF was derived to predict the composite endpoints. But the ROC curve was not significant for hs-cTnT to predict the composite endpoints in NIHF. Moreover, the effect of hs-cTnT on cardiovascular events between IHF and NIHF was not reported in the previous literature. Definitely, hs-cTnT representing myocardial injury was an indicator of re-hospitalization and death in HF patients. More studies should be focused on the prognostic role of hs-cTnT in NIHF and explore the difference in hs-cTnT between IHF and NIHF to treat early and improve prognosis in HF.
We mainly aimed to determine the prognostic role of hs-cTnT between IHF and NIHF; therefore, the HF patients included were only those in whom HF was caused by ischemic heart disease or non-ischemic heart disease; we eliminated those with mixed heart disease. Due to the strict selection criteria, the amount of data in the study is relatively small and may not fully reflect the value of hs-cTnT. In our study, myocardial ischemia was evaluated by CAG or CCT, which was used for the assessment of macrovascular coronaries but not sufficiently for cardiac microcirculation and functional ischemia. In addition, although sepsis and chronic renal insufficiency were excluded in this study, it is not clear whether general infection and transient renal insufficiency may interfere with the results of this study. The follow-up time was relatively short as it has not yet reached 3–5 years. Therefore, this study cannot fully reflect the long-term prognoses of patients with HF.
Conclusions
Hs-cTnT varied significantly over time, showing an increasing trend in NIHF while first increasing and then decreasing in IHF. Hs-cTnT was independently related to re-hospitalization rate for HF and all-cause mortality in patients with HF, which could play an important role in prognosis of HF.