Introduction
Vitamin D (Vit. D) plays an important role in regulating calcium (Ca+) absorption, and only 15% of the Ca+ is absorbed in vitamin D deficiency [1]. Vitamin D helps the parathyroid hormone (PTH) in skeletal mineralisation [1].
The parathyroid hormone is a single-chain hormone containing 84 amino acids of 9.5 KD molecular weight [2], secreted from the parathyroid glands (PTGs), and it is responsible for the Ca+ haemostasis [2].
The parathyroid hormone stimulates the conversion of 25(OH)Vit. D to 1.25(OH)Vit. D in the kidney, and the 1.25(OH)Vit. D increases the intestinal absorption of Ca+ [2].
Parathyroid hormone secretion is regulated by the feedback loops (negative and positive loops), serum Ca+ phosphorus, and vitamin D [3]. The parathyroid hormone acts as calcitonin antagonist on the renal tubules, and it plays a crucial role in bone turnover (BTO) [4].
The parathyroid glands contain vitamin D receptors (VDRs), and stimulation of the VDRs on the PTGs reduces the PTH secretion [2].
An inverse association between the PTH and vitamin D was reported previously [1, 5] and the 25(OH)D intake reduces the serum PTH [6].
Adolescents have elevated levels of circulating BTO markers, reflecting the period of accelerated growth [7].
A population-based national survey showed that the bone mineral density (BMD) of the hip increased when serum 25-hydroxy vitamin D (25(OH)D) is > 32 ng/ml (80 nmol/l) [8].
Vitamin D deficiency leads to secondary hyperparathyroidism with subsequent increased BTO and bone loss [2]. Serum 25(OH)D is an accurate predictor of the actual vitamin D status [9].
Considering the hypothesis of decreased Ca+ absorption [1] and secondary hyperparathyroidism associated with vitamin D deficiency [2], the current study aimed to detect the relationship between 25(OH)D and adolescents’ PTH and BMD.
Material and methods
Two hundred adolescent girls were recruited for this cross-sectional comparative study, which was conducted in the Republic of Kazakhstan (RZ) over 2 years (2021–2022), to detect the relationship between 25(OH)D and adolescents’ PTH and BMD.
Adolescents were recruited for the current study after West Kazakhstan University (WKU) Ethics Committee approval (No. 10 dated 4 October 2020) and after informed consent from the adolescents and their parents/guardians in accordance with the Helsinki declaration.
After detailed history and examination, the studied adolescents’ BMI was calculated, followed by a pelvic sonography to rule out any pelvic pathology.
Inclusion criteria included girls (> 12 and < 18 years old), with regular menstrual flow (within > 21 and < 35 days), and normal BMI > 18.5 and < 24.9 kg/m2, without any known chronic and/or endocrine disorders.
Exclusion criteria included the following:
Adolescents < 12 or > 18 years old;
BMI < 18.5 kg/m2 (underweight), BMI 25–29.9 kg/m2 (overweight), or BMI > 30 kg/m2 (obese) [10, 11];
Irregular menstrual flow;
Medical disorders (i.e. diabetes or hypertension);
Endocrine disorders (i.e. polycystic ovary syndrome, Cushing’s syndrome/disease [11], thyroid, or hyperprolactinaemia);
Those who received exogenous hormones, vitamin D, or steroids within the last year before inclusion in the current study and/or refused to give consent.
Diabetes defined according to the American Diabetic Association as a metabolic disorder characterised by hyperglycaemia (either from insulin deficiency or defective insulin action) and diagnosed when HbA1C and fasting plasma glucose were 6.5% and ≥ 126 mg/dl, respectively [12].
Hypertension diagnosed when the systolic and/or diastolic blood pressure were ≥ 140 mm Hg and/or ≥ 90 mm Hg, respectively (on 2 different occasions) [13]. Polycystic ovary syndrome was diagnosis based on the ESHRE/ASRM criteria [10, 11].
Adolescents with suspected Cushing’s syndrome/disease were subjected to dexamethasone suppression test [11].
Adolescents’ blood samples were collected to measure the thyroid stimulating hormone (i.e. to exclude thyroid disorders) [14], prolactin (i.e. to exclude hyperprolactinemia) [15], HbA1C, PTH, and 25(OH)D.
The adolescents’ BMD and the T-score were evaluated at 2 anatomical sites (i.e. lumbar spine [L1-L4] and neck of right femur) using the DEXA (Lunar Corp., Madison). The T-score was calculated based on the normal reference for matched adolescents’ subjects (T-score < –1 means osteopaenia, and T-score ≤ –2.5 means osteoporosis) [2].
The serum PTH was measured using the radioimmunoassay techniques [16], and normal adolescents’ PTH ranges from 15 to 31 pg/ml [17].
Serum 25(OH)D is an accurate predictor of the actual vitamin D status (25(OH)D > 30 ng/ml means normal vitamin D, while 25 (OH)D < 20 ng/ml means vitamin D deficiency) [9].
The adolescents’ blood was centrifugated and stored at –20°C for the quantitative 25(OH)D evaluation. The 25(OH)D level was measured using an Architect kit (Abbott, Longford, Ireland). The Architect 25(OH)D evaluation is a delayed 1-step chemiluminescence microparticle evaluation using the Architect 25(OH)D Reagent Kit [1].
The studied adolescents were classified according to their serum 25(OH)D into 2 groups: a 25(OH)D- deficient group (study group; 25(OH)D < 20 ng/ml) and normal controls (25(OH)D > 30 ng/ml).
Statistical analysis
Student’s t-test was used for analysis of the adolescents’ variables, and p < 0.05 was considered significant. The correlation analysis (Pearson’s correlation) was used to detect the relationship between 25(OH)D and adolescents’ PTH and BMD.
Study sample
G Power 3.1.9.7 with 0.05 probability, 0.95% power, and 0.5 sample size was used to calculate the sample size [18, 19].
Ethical considerations
Results
Two hundred adolescent girls between 12 and 18 years of age were included in this cross-sectional comparative study to detect the relationship between 25(OH)D and adolescents’ PTH and BMD (Figs. 1–3).
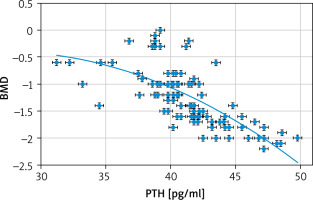
Fig. 3
Correlation between parathyroid hormone and bone mineral density
BMD – bone mineral density, PTH – parathyroid hormone
The studied adolescents were classified according to their serum 25(OH)D into 2 groups: a 25(OH)D- deficient group (study group; 25(OH)D < 20 ng/ml) and normal controls (25(OH)D > 30 ng/ml).
No statistical difference was found between the 25(OH)D-deficient group and controls regarding the mean age (15.04 ±1.2 years vs. 14.98 ±1.4, respectively) (p = 0.94), weight (58.7 ±2.4 kg vs. 58.01 ±3.1, respectively) (p = 0.99), height (157.8 ±1.7 cm vs. 157.8 ±2.3, respectively) (p = 0.99), and BMI (23.5 ±0.79 kg/m2 vs. 23.2 ±1.03, respectively) (p = 0.99) (Table 1).
Table 1
Adolescents’ characteristics, 25-hydroxy vitamin D, parathyroid hormone, lumbar and hip bone mineral density
The 25(OH)D was statistically lower in the 25(OH)D-deficient group than in the normal controls (13.83 ±2.5 ng/ml vs. 35.62 ±1.03, respectively), (p = 0.003; 95% CI: –22.4, –21.8, –21.2).
The parathyroid hormone was statistically higher in the 25(OH)D-deficient group than in the normal controls (41.3 ±3.4 pg/ml vs. 21.1 ±2.8) (p = 0.02; 95% CI: 19.3, 20.2, 21.1), and the BMD was statistically lower in the 25(OH)D-deficient group than in the normal controls (–1.25 ±0.5 vs. 0.3 ±0.4) (p = 0.01, 95% CI: –1.7, –1.6, –1.4) (Table 1).
Discussion
Vitamin D plays an important role in regulating Ca+ absorption, and only 15% of the Ca+ is absorbed in vitamin D deficiency [1]. Vitamin D helps the PTH in skeletal mineralisation [1], and 25(OH)D intake reduces the serum PTH [6].
Adolescents have elevated levels of the BTO markers, reflecting the period of accelerated growth [7]. Vitamin D deficiency leads to secondary hyperparathyroidism with subsequent increased BTO and bone loss [2].
Considering the hypothesis of decreased Ca+ absorption [1] and secondary hyperparathyroidism associated with vitamin D deficiency [2], the current study aimed to detect the relationship between 25(OH)D and adolescents’ PTH and BMD.
The studied adolescents were classified according to their serum 25(OH)D into 2 groups: a 25(OH)D- deficient group (study group; 25(OH)D < 20 ng/ml) and normal controls (25(OH)D > 30 ng/ml).
Correlation between parathyroid hormone and both 25(OH)D and bone mineral density
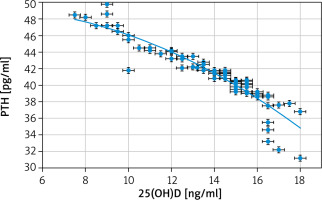
The parathyroid hormone was statistically higher in the 25(OH)D-deficient group than in the normal controls (41.3 ±3.4 pg/ml vs. 21.1 ± 2.8) (p = 0.02). The parathyroid hormone in this study had significant negative correlations with both 25(OH)D (r = –0.9175; p < 0.00001) and BMD (r = –0.7006; p < 0.00001).
Similarly, a cohort study [2] and a cross-sectional study (including 102 patients) found a negative correlation between PTH and both the 25(OH)D and BMD at the lumbar spine and hip [20].
Vitamin D deficiency is associated with secondary hyperparathyroidism with subsequent and bone loss [2].
Similarly, Martins et al. [5] also found a negative relationship between 25(OH)D and PTH.
Chen et al. [21] also reported an inverse relationship between 25(OH)D and PTH over the whole spectrum of 25(OH)D levels.
Moreover, Sai et al. [1] reported an inverse relationship between 25(OH)D and PTH and a positive relationship between 25(OH)D below 18 ng/ml and BTO markers (i.e. osteocalcin).
Sneve et al. [22], in a cross-sectional study, found that serum PTH had an inverse association with BMD at the hip.
A meta-analysis investigating the effect of vitamin D intake in primary hyperparathyroidism found that vitamin D intake significantly reduces PTH [23].
Additionally, a systematic review found that vitamin D intake significantly reduces the PTH in primary hyperparathyroidism [24].
Correlation between 25(OH)D and bone mineral density
A cohort study [2] and a cross-sectional study (including 102 patients) found no relation between 25(OH)D and BMD [20].
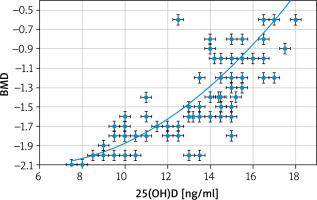
However, this study found that BMD was statistically lower in the 25(OH)D-deficient group than in normal controls (–1.25 ±0.5 vs. 0.3 ±0.4) (p = 0.01). 25(OH)D had a significant positive correlation with the studied adolescents’ BMD (r = 0.756; p < 0.00001).
Kamineni et al. [25] examined 100 menopausal women and found that the risk of bone loss and osteoporosis increased with vitamin D deficiency. Wang et al. [26], in a cross-sectional study, found that vitamin D intake could reduce bone loss and osteoporosis in menopausal women. Moreover, a population-based national survey showed increased BMD at serum 25(OH)D > 32 ng/ml (80 nmol/l) [8].
This relation between vitamin D and BMD can be explained by the secondary hyperparathyroidism caused by vitamin D deficiency. Hyperparathyroidism increases the BTO with subsequent increased bone loss and decreased BMD [2].
A review of RCTs found an association between the 25(OH)D levels and healthy bones [27]. The same review also found that vitamin D3 supplementation (> 700 IU/day) plus Ca+ had a beneficial outcome on BMD compared to placebo [27].
This study was the first cross-sectional comparative study conducted in the RZ to detect the relationship between 25(OH)D and adolescents’ PTH and BMD. This study found that PTH was statistically higher in the 25(OH)D-deficient group than in the normal controls, and the PTH had significant negative correlations with both 25(OH)D and BMD. This study also found that the adolescents’ BMD was statistically lower in the 25(OH)D-deficient group than in the normal controls, and it had a significant positive correlation with the adolescents’ 25(OH)D.
Failure to detect the suitable serum 25(OH)D level for optimal bone health (because of the cross-sectional nature of the study) was the only limitation of the current study.
Further larger studies are needed to detect the suitable serum 25(OH)D level for optimal adolescents’ bone health.