Introduction
Postmenopausal abnormal uterine bleeding (AUB), which is present in 5% of women, is defined as pathological bleeding from the genital tract that occurs at least 12 months after a woman’s last menstruation [1]. Vaginal bleeding may be the presenting sign of endometrial cancer, which is the most common malignancy of the female reproductive tract in developed countries [2]. The incidence of postmenopausal AUB decreases with age, while the possibility of cancer as the underlying cause escalates from approximately 1% at the age of 50 to 25% in women aged 80 years [3]. Factors increasing the risk of malignancy in endometrial tissue include obesity, diabetes mellitus, arterial hypertension, nulliparity and unopposed estrogen therapy [4].
Dilation and curettage, an endometrial aspiration (office biopsy) and hysteroscopy (hysteroscopic guided biopsy) are used to obtain a tissue sample for the diagnosis and treatment of endometrial pathologies. However, endometrial thickness measurement using transvaginal ultrasound is a common screening method for triaging AUB in postmenopausal women [2]. In case of endometrial thickness > 4 mm endometrial sampling is indicated to exclude malignancy. In women with endometrial echo ≤ 4 mm the probability of endometrial malignancy is considered to be low; therefore refraining from endometrial biopsy or curettage is sometimes considered justified [5].
The aim of this study was to investigate the risk of developing endometrial malignancy in women with postmenopausal bleeding with endometrial thickness ≤ 4 mm.
Material and methods
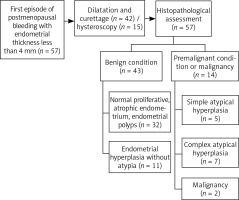
The retrospective study included 57 Caucasian women presenting with postmenopausal bleeding at the Gynecological Endocrinology Department, Jagiellonian University Medical College, from January 2014 to December 2016. Postmenopausal bleeding was defined as one-time or recurrent vaginal bleeding after at least 12 months of amenorrhea. Only women with endometrial thickness ≤ 4 mm were included in the analysis. Women with self-reported history of breast cancer with or without therapy with tamoxifen (n = 1) and use of anticoagulants (n = 3) were excluded. The diagnostic work-up consisted of a double-layer measurement of the endometrium using transvaginal ultrasound and a cervical smear. The endometrial thickness was measured at its thickest point from the anterior to the posterior in the sagittal plane of the uterus. An endometrial sample was obtained via hysteroscopic biopsy or dilatation and curettage. Hysteroscopic biopsy was more often performed among women with suspected polyps as well as among women with contraindications to general analgesia. Histological assessment was performed by the pathologists from the Department of Pathomorphology, Jagiellonian University Medical College.
Normal proliferative and atrophic endometrium as well as endometrial polyps were classified as benign. Endometrial hyperplasia without atypia was considered as without the need for further followup, despite the slightly increased risk of endometrial cancer. Both simple and complex atypical hyperplasia were classified as premalignant conditions. Hysterectomy was proposed to women with atypical hyperplasia and endometrial cancer.
Body mass index (BMI) was calculated by dividing weight in kilograms by square of height in meters. Information about concomitant diseases such as diabetes mellitus, arterial hypertension, hypercholesterolemia, as well as number of pregnancies, was obtained during a medical interview. Smoking was specified as being a current smoker. Hormone therapy (HT) users were defined as taking HT currently or in the perimenopausal period.
The Ethics Committee at Jagiellonian University Medical College approved the study, and participants provided informed consent in accordance with the Declaration of Helsinki.
Statistical analysis
Data were expressed as mean ± standard deviation or median (interquartile range) unless otherwise stated. The Kolmogorov-Smirnov test was used to assess conformity with a normal distribution.
A 2-sided p-value of less than 0.05 was considered statistically significant. Statistical analyses were performed with Statistica 12 (StatSoft, Tulsa, OK, USA).
Results
The final analysis included 57 postmenopausal women aged from 42 to 69 years.
The characteristics of the studied group are shown in Table 1. Both groups with benign and malignant (or premalignant) histologic results were similar regarding demographic variables. Forty-three women (75.4%) had their last menstruation between the ages of 45 and 55. Only 13 women (22.8%) were diagnosed with obesity (BMI > 30 kg/m2) (data not shown).
Table 1
Baseline characteristics of postmenopausal women with abnormal uterine bleeding
The two groups were similar regarding incidence of diabetes mellitus, arterial hypertension, hypercholesterolemia, smoking or use of HT (Table 1). All the participants had at least 1 child (data not shown).
Dilatation and curettage (73.7%) or hysteroscopy (26.3%) were performed to obtain endometrial samples. (Fig. 1). On histopathology, the majority of women (75.4%) had benign results, either normal proliferative and atrophic endometrium (56.1%) or hyperplastic endometrium without atypia (19.3%). Endometrial hyperplasia with atypia was detected in 12 (21.1%) women. Two women (3.5%) were diagnosed with endometrial malignancy; their ages were 60 and 54 years, and they were diagnosed with clear cell adenocarcinoma and uterine sarcoma, respectively.
We also evaluated the number of patients according to histopathology and endometrial thickness in transvaginal ultrasonography (Table 2). As expected, the largest number of women with abnormal histology were in the group with the widest endometrium (by 9.7%) and had a mean endometrium thickness of 3.5 mm (data not shown). None of the participants from this group had endometrium thickness less than 2 mm. No similar changes were found in the benign histopathology group.
Table 2
Histopathological assessment of endometrial samples obtained via hysteroscopy or curettage in postmenopausal women with abnormal uterine bleeding
All women with both endometrial atypical hyperplasia and malignancy were treated with simple hysterectomy and bilateral oophorectomy. The final diagnosis of the hysterectomy specimen performed among 12 women with atypical endometrial hyperplasia revealed that only 1 woman (8.3%) had endometrial cancer, which accounted for 10% of women with atypical hyperplasia with endometrial thickness between 3.1 and 4.0 mm (Table 3). The assessment in the hysterectomy material for clear cell carcinoma showed focal extension and the staging was Ib (FIGO grade 1). Histopathological evaluation of uterine sarcoma demonstrated low-grade endometrial stromal sarcoma (FIGO IA).
Discussion
Based on histology and clinical outcomes, endometrial cancers have been divided into two types. Type I tumors are associated with unopposed estrogen stimulation, and are often preceded by endometrial hyperplasia, whereas type II tumors are predominantly serous carcinomas and are commonly described as estrogen independent, arising in atrophic endometrium and deriving from intraepithelial carcinoma, a precancerous lesion [6]. In our study more than 20% of women were diagnosed with atypical hyperplastic endometrium, which is a precursor of type I endometrial cancer.
At present, transvaginal ultrasonography is the initial imaging procedure of choice for evaluating AUB in postmenopausal women [7]. The possibility of abnormal (premalignant or malignant) pathology is very limited in women with endometrial thickness less than 5 mm [8, 9].
The finding that 24.6% of women in the whole group had an abnormal histological diagnosis strongly indicates that an endometrial thickness ≤ 4 mm cannot be ignored and, where possible, a histological diagnosis must be sought. Our results confirm that endometrial cancers can indeed occur despite reassuring appearances of the endometrium on an ultrasound scan. Büyük et al. also detected endometrial malignancies in women with an endometrial stripe as thin as 3 mm [10]. However, a meta-analysis of 35 published studies showed that in 5 892 women with postmenopausal bleeding who had endometrial thickness of 5 mm or more 95% of all endometrial malignancies were identified, whereas in women with endometrial thickness less than 4 mm cancer was diagnosed in only 1% [11, 12]. Karlsson et al. reported that the risk of finding endometrial pathology in women with endometrial thickness ≤ 4 mm is 5.5% [13]. Moreover, Goldstein indicated that in women with postmenopausal bleeding, biopsy is not required when endometrial thickness is ≤ 4 mm [14]. Clark et al. found a strategy with TVS as the screening test with a cut-off of 4 mm followed by endometrial sampling being the most effective [15]. Ferrazzi et al. in a multicenter study of 930 postmenopausal women with AUB found 2 cases of endometrial cancer when endometrial thickness was ≤ 4 mm and 4 participants with a malignant condition when endometrial thickness was ≤ 5 mm [16]. Gull et al. in two studies where women with postmenopausal AUB and the endometrial echo ≤ 4 mm were included confirmed only 1 (0.6%) endometrial cancer in the first study (163 women involved), whereas no cases of malignant condition were found in the second study (394 women screened) [17, 18]. In another study where 97 women with postmenopausal AUB were recruited, no malignancies were confirmed with narrow endometrium ≤ 5 mm [19]. Interestingly, none of the above studies evaluated the correlation between the risk factors of endometrial cancer and endometrial thickness. Based on the American College of Obstetricians and Gynecologists (ACOG) Committee Opinion, endometrial sampling is not required in women with postmenopausal AUB with an endometrial thickness of 4 mm or less because of a very low risk of uterine malignancy in these patients [20]. However, this committee also stated that when bleeding persists despite negative initial evaluation, additional assessment is indicated. Nevertheless, in our study we decided to perform histological examination of endometrial tissue despite the lack of obvious features of malignancy in transvaginal ultrasonography examination.
The main purpose of endometrial sampling is to exclude endometrial cancer. The accuracy of curettage compared to both hysteroscopy and Pipelle endometrial biopsy in differentiation between benign and malignant condition is uncertain. Endometrial biopsy is a less invasive procedure and it can be performed in the office. Nevertheless, it may not give a final diagnosis, especially when an insufficient amount of tissue is collected, which is present in women with endometrial thickness ≤ 4 mm [21]. That is why, in our study, we did not use that procedure to identify the cause of postmenopausal AUB. However, both curettage and Pipelle sampling have been reported to yield equal rates of cancer detection in patients with abnormal uterine bleeding [22]. Hysteroscopy is better when imaging of the uterine cavity is required in the case of diagnosis of focal lesions and it allows removal of polyps at the time of diagnosis. However, not all premalignant lesions can be visualized by hysteroscopy [23].
The article has several limitations. The population of women with postmenopausal AUB and narrow endometrium was limited. The first possible cause is that women with endometrial thickness ≤ 4 mm and postmenopausal abnormal uterine bleeding do not undergo histopathological assessment of endometrial sampling obtained during curettage or hysteroscopy until the endometrial echo increases in size. The next possible cause of the limited population in this study is histopathological assessment of endometrial sampling obtained via Pipelle endometrial biopsy outside the hospital and lack of access to these results.
Finally, we did not follow the women with normal histopathologic results to assess the possible cause of AUB.
Conclusions
In conclusion, this study found that women with endometrial echo ≤ 4 mm are less likely to be associated with a malignant condition. However, the role of histopathological evaluation in postmenopausal women suffering from AUB with endometrial thickness ≤ 4 mm cannot be undermined, especially in patients at high risk of endometrial cancer. Nevertheless, considering the rising incidence of endometrial cancer, more research on this subject is needed, using larger cohorts of women with postmenopausal AUB with thickened endometrium on transvaginal ultrasonography to evaluate the effectiveness and diagnostic accuracy of endometrial sampling. Therefore, we recommend a change in the cut-off to 2 mm in routine practice.