Introduction
Lung cancer is an important health problem with an increasing incidence. In most European countries, lung cancer has increased so dramatically that it may be considered one of the major health problems in the last century [1]. The most common causes of cancer-related death are cancers of the lung and bronchus (24%), prostate (10%) and colorectum (9%) in men, and lung and bronchus (23%), breast (15%), and colorectum (8%) in women [2]. Although lung cancer has long been characterized by late-stage diagnosis and poor survival, encouraging results have been achieved for lung cancer screening in high-risk populations in the last decade and there has been significant progress in systemic treatments for molecular subgroups of patients with advanced disease. Furthermore, within the last ten years, new molecular targets have emerged, next-generation drugs with more specific target effects have been introduced, and targeting specific resistant mutations is expected to advance the treatment of lung cancer by creating a chronic therapeutic pathway [3]. This bibliometric study demonstrates the development of lung cancer treatment over the years.
Bibliometric studies represent an important study type showing the trend topics in a given field. Numerous medical and surgical specialists have published the most cited articles in their specialties in the form of bibliometric analysis such as general surgery [4], anesthesiology [5], orthopedics [6], otolaryngology [7], radiology [8] and plastic surgery [9]. The first bibliometric analysis was penned by Garfield and published in JAMA in 1987 [10]. He also continued with new bibliometric studies in different fields of medical science.
The purpose of our study was to identify and analyze the 100 most cited lung cancer articles published in biomedical literature in the last 44 years. We determined the number of citations with ranking, average citations per year (ACY), citations and publications by year, publishing journal, institution and country of origin, the most common subject of frequently cited articles, authorship status of classical papers and correlation analyses between citation, ACY, Impact Factor (IF) and length of time since publication in years.
Material and methods
Study design
Study type: retrospective clinical study, Level of evidence: 3 or Group B (Scottish Intercollegiate Guidelines Network; SIGN) [11].
Data collection and inclusion criteria: In this paper reporting a bibliometric citation analysis, data were obtained from Thomson Reuters’ WoS Core Collection database (Philadelphia, Pennsylvania, USA) and PubMed (US National Library of Medicine-National Institutes of Health). We accessed the WoS database (accessed: 15.07.2019) using the keyword “lung cancer” between 1975 and 2019. We identified 240,701 articles and conducted an analysis of the top 100 cited articles among these hits shown in Table 1 [12–111]. Articles not relevant to lung cancer were excluded from our study and we included original research articles, editorials, correspondences, review articles and case reports. We also utilized the PubMed database to obtain additional data related to the study. Two of the authors (NSS and EC) independently identified T100 with consensus. The difference in time since publication among the top 100 articles may cause a bias as older articles may be more likely to have obtained more citations owing to a longer citable period. The Web of Science, Citation Report feature displays bar charts for the number of items published each year and calculates the average number of citations per year per publication. Due to this bias, we used the ACY for each article.
Table 1
The top 100 cited articles in lung cancer
Statistical analysis
A commercial software (SPSS version 16.0, SPSS, Chicago IL, USA) was used for the statistical analysis. The Kolmogorov-Smirnov test was used to analyze the normal distribution of data. Spearman’s correlation was used to evaluate the associations between citation, ACY, IF and length of time since publication. A p-value < 0.05 was accepted as statistically significant.
Ethical statement
All authors declare that the study was conducted according to the principles of the World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. This study did not require approval from an ethics committee as it was designed as a bibliometric analysis or citation analysis of existing published classical studies.
Results
We identified 240,701 articles from 1975 to 2019. The language was English for all articles. The 100 most cited articles in lung cancer are listed in Table 1, arranged in descending order according to the number of times cited. The number of citations ranged from 7751 to 889, and the mean number of citations per article was 1879.82 ±1264.78 (range: 7751–889). We found that the most cited article (times cited: 7751) on lung cancer was a study by Lynch et al. with the following title: “Activating mutations in the epidermal growth factor receptor underlying responsiveness of nonsmallcell lung cancer to gefitinib” published in N Engl J Med 2004; 350: 2129-1239. The least cited article (times cited: 889) on lung cancer was penned by Imielinski et al. with the following title: “Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing” and published in Cell 2012; 150: 1107-1120. Additionally, we determined that there were 84 articles that got more than 1000 citations and the article with the highest ACY was the article that ranked 16 in the T100 list. The article with the highest ACY was a randomized phase 3 trial by Borghaei et al., titled “Nivolumab versus docetaxel in advanced nonsquamous non-smallcell lung cancer” and published in N Engl J Med 2015; 373: 1627-1639. The highest number of citations was seen in 2017 with 18,393 citations while the highest number of publications was seen in 2005 with 12 publications.
The oldest article was a review published in Nature 1985; 316: 823-826 titled “Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer” by Cuttitta et al. with 1280 citations and ACY 36.57 ACY. The newest study in the T100 list was a phase 3 trial conducted by Rittmeyer et al. published in Lancet 2017; 389: 255-265 with the following title: “Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial”, with 926 citations and ACY 308.67.
In the T100 list, 82 were clinical studies and 18 were experimental studies. The 82 clinical articles included 42 randomized controlled studies, 8 review articles, 4 meta-analyses, 2 case reports and other clinical studies. Fifty-nine of these 82 clinical articles were treatment-based studies. The treatment-based studies are classified in Table 2 according to the level of evidence.
Table 2
Type of treatment and level of evidence of the treatment based clinical articles (n = 59)
| Treatment | Level 1 | Level 2 | Level 3 | Level 4 |
|---|---|---|---|---|
| EGFR mutations | 17 | – | 6 | – |
| Chemotherapy | 19 | – | – | 1 |
| Palliative care | 1 | – | – | – |
| Immunotherapy | 5 | 1 | 1 | – |
| ALK mutations | 2 | – | 1 | – |
| Radiotherapy | 3 | 1 | 1 | – |
While 32 of these articles were published in NEJM, 16 were published in the Journal of Clinical Oncology, 7 in The Lancet, 7 in Science, etc. (Table 3).
Table 3
List of journals with published articles
| Journal | Number of articles | Impact Factor* | Quartile score** |
|---|---|---|---|
| New England Journal of Medicine (NEJM) | 32 | 79.258 | Q1 |
| Journal of Clinical Oncology | 16 | 26.303 | Q1 |
| Lancet | 7 | 53.254 | Q1 |
| Science | 7 | 41.058 | Q1 |
| Lancet Oncology | 5 | 36. 418 | Q1 |
| Journal of the National Cancer Institute (JNCI) | 4 | 11.238 | Q1 |
| Cancer Research | 3 | 9.13 | Q1 |
| Cell | 3 | 31.398 | Q1 |
| Chest | 3 | 7.652 | Q1 |
| Nature | 3 | 41.577 | Q1 |
| Proceedings of the National Academy of Sciences of the United States of America | 3 | 9.504 | Q1 |
| Journal of the American Medical Association (JAMA) | 2 | 47.661 | Q1 |
| Journal of Thoracic Oncology | 2 | 10.336 | Q1 |
| American Review of Respiratory Disease | 1 | 6.27 | Q1 |
| British Medical Journal (BMJ) | 1 | 2.12 | Q1 |
| Cancer Cell | 1 | 22.844 | Q1 |
| Cell Death & Differentiation | 1 | 8.000 | Q1 |
| Genes & Development | 1 | 9.462 | Q1 |
| Mayo Clinic Proceedings | 1 | 7.199 | Q1 |
| Nature Reviews Cancer | 1 | 42.784 | Q1 |
| Oncogene | 1 | 6.854 | Q1 |
| PLOS Medicine | 1 | 11.675 | Q1 |
| Science Translational Medicine | 1 | 16.710 | Q1 |
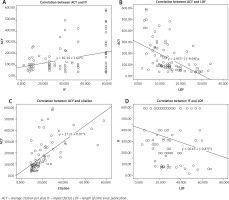
All of the T100 articles were published across 23 different journals. Eighty-five of the T100 articles were published in 14 journals that had IF ≥ 10.336. We determined that the mean IF of these 23 journals was 23.42 ±19.90 (range: 79.26–2.12) (according to Clarivate Analytics, 2017). The “Quartile Score” category was Q1 for all the journals (according to SCImago Journal and Country Rank, 2019). Most of the articles were published in NEJM, and NEJM was also the journal with the highest IF. The correlation analysis for the number of citations, ACY, IF and length of time since publication parameters in the T100 list revealed a positive correlation between citation and ACY (r = 0.744, p = 0.00) and between ACY and IF (r = 0.236, p = 0.018), whereas a negative correlation was observed between ACY and length of time since publication (r = –0.562, p = 0.00) and between IF and length of time since publication (r = –0.266, p = 0.008). There was no correlation between citation and length of time since publication or between citation and IF (Fig. 1).
Fig. 1
Correlation analysis for the citation numbers, ACY, IF, length of time since publication parameters
According to the geographic origin of the T100 list, the USA (n = 74) was the most contributing country, followed by Japan and Canada (Table 4). We determined that the most commonly listed institution was the University of Harvard (USA), which was listed 27 times in the top 100 cited articles (Table 5). Moreover, 11/19 of the institutions that published eight or more publications were found to be in USA.
Table 4
Geographic origin of the top 100 articles
Table 5
Institutions of origin with 8 or more of the top 100 cited articles
| Rank | Institution | Number* |
|---|---|---|
| 1 | Harvard University | 27 |
| 2 | VA Boston Healthcare System | 26 |
| 3 | Dana Farber Cancer Institute | 17 |
| 4 | University of Texas System | 17 |
| 5 | Memorial Sloan Kettering Cancer Center | 16 |
| 6 | University of California System | 16 |
| 7 | UT MD Anderson Cancer Center | 16 |
| 8 | Massachusetts General Hospital | 15 |
| 9 | Unicancer | 15 |
| 10 | Vanderbilt University | 15 |
| 11 | University of Toronto | 10 |
| 12 | Princess Margaret Cancer Centre | 9 |
| 13 | Ruprecht Karls University Heidelberg | 9 |
| 14 | University Health Network Toronto | 9 |
| 15 | Astrazeneca | 8 |
| 16 | Gustave Roussy | 8 |
| 17 | National Institutes of Health NIH USA | 8 |
| 18 | Samsung Medical Center | 8 |
| 19 | Sungkyunkwan University | 8 |
| 20 | University of California Los Angeles | 8 |
It was seen that 3 authors were the first author in more than one article in the T100 list’s top 12 authors (Table 6). Herbst RS contributed to 8 articles and was the first author in 4 of them. Janne PA, Johnson BE and Johnson DH also contributed to 8 articles. However, Herbst RS had the highest number of articles as first author. The “Web of Science” category analysis of the T100 in the field of the lung cancer revealed that these articles ranked under general internal medicine (n = 47), oncology (n = 33), multidisciplinary sciences (n = 13), cell biology (n = 8) and respiratory system (n = 6) as the most featured branches.
Discussion
Lung cancer is the major cause of cancer-related deaths worldwide. There are two main types of this cancer: small-cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). NSCLC accounts for 80% of all lung cancers. Despite the advances in surgical methods and advances in radiotherapy and chemotherapy, non-small-cell lung cancer continues to account for the majority of lung cancers and is associated with a 5-year survival rate of 15% [112].
There have been significant advances in the treatment of lung cancer in the last 40 years, and this is reflected in the scientific literature. A better understanding of disease progression coupled with targeted immunological therapies has led to increased survival rates.
We found that in our top 100, 28% of the articles were less than 10 years old while 72% of them were older than 10 years. Articles with a higher number of citations are indeed expected to be older. Year of publication and number of citations for an article are closely linked, and the number of citations grows over time. Needless to say, citation is an important metric, which shows the quality and attractiveness of an article; however, a certain amount of time should be allowed to pass after the publication of an article for it to reach a higher number of citations. For that reason, number of citations alone is inadequate to determine the quality of an article. In this study, ACY was used to eliminate the time bias when evaluating older articles against newer articles. Of the T100, 18% were comparative studies, and there were 2 case reports in the T100 list. The two case reports were published in 2005. One of them was published in NEJM (times cited: 2549), and the other in Plos Med (times cited: 2073). Both were about EGFR mutations. It is noteworthy that a case report receives such a high number of citations. This may be due to the fact that EGFR mutations were popular in the 2000s. In the T100, 29% of the articles were noted to concern erlotinib (anti-EGFR), gefitinib (anti-EGFR) and EGFR mutations. The 1st study with the highest number of citations was a study related to EGFR mutations, showing that EGFR mutations play an important role in the development stages of lung cancer treatments.
Immunotherapy has become one of the most promising treatments for several human cancers. In fact, James P. Allison and Tasuku Honjo were awarded with the Nobel Prize in medicine for their research on immune checkpoint blockade [113, 114]. As a result, the immune check-point inhibitor (ICPI) may be regarded as an immunotherapy modality that started a new era in cancer treatment and remains a new trend topic. Especially in advanced non-small cell lung cancer (NSCLC), significant improvement has been observed in survival results with anti-PD-1 and PDL-1 drugs compared to chemotherapy. That shows the changing trends in cancer immunotherapy during the last decade. We can also see studies on immunotherapy in the T100 list. The most cited immunotherapy-related study in T100 was published in 2015 and received 2966 citations (ACY 593.2). It was published in N Engl J Med 2015; 373: 123-135 by Brahmer et al. with the following title: “Nivolumab versus docetaxel in advanced squamouscell non-smallcell lung cancer”. This study currently remains a new study of only 4 years old, and despite being a very young article, the number of citations it has received shows that the study in question involves a very important innovation. Moreover, this article has the highest ACY score in the T100 list. This shows that scientists are currently focused on immunotherapy. There are only 7 studies about immunotherapy in the T100, and the newest article in the T100 was published in Lancet 2017; 389: 255-265 by Rittmeyer, titled “Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial”. It is only a 2-year-old article; however, it has 926 citations with an ACY score of 308.67. When we list the articles based on ACY scores in descending order, the first 4 articles are immunotherapy-related and recent articles.
The correlation analysis showed a positive correlation between citation and ACY and between ACY and IF, whereas a negative correlation was found between ACY and length of time since publication and between IF and length of time since publication. This indicates that articles with high ACY scores have been published in journals with a high IF. Furthermore, younger articles have higher ACY scores and have been published in journals with a higher IF.
When we looked at the T100 list, another point of interest also caught our attention: there were very few articles related to small-cell lung cancer (SCLC). Only 3 articles were on small-cell lung cancer [115–117]. This either means that there has not been any significant advance in SCLC or scientists are less interested in this topic.
Conclusions
To the best of our knowledge, this is the first report of a citation analysis of lung cancer in the English literature. The first 100 articles in our analysis not only identify landmark articles that have the greatest impact on lung cancer research, but also acknowledge the most productive authors and institutions that contributed to the list with their articles. Oncology is a developing field in science, and we have seen its evolution through the treatment of lung cancer over the years. Briefly, bibliometric analyses for different medical disciplines and sub-specialties demonstrate the improvements in a given field from a nominative perspective. The present bibliometric citation analysis on lung cancer has covered several scientific fields, and we believe it enables the systematic identification of true landmark publications as well as the distribution of citations of these publications by year, main topic, institution, scientific journal, level of evidence, and correlation analysis, thereby providing a substantial contribution for oncological research.







