Introduction
Osteoporosis is a skeletal disorder characterized by decreased bone mass and microarchitectural deterioration of bone tissue, resulting in increased fragility and a heightened risk of fractures. Fracture management in osteoporotic patients poses significant challenges due to biomechanical limitations, including impaired healing associated with excessively rigid or unstable fixation, reduced screw anchorage in osteoporotic bone, and premature fatigue at the implant-bone interface. These factors contribute to implant loosening and fixation failure in osteoporotic fractures [1]. The compromised mechanical properties of osteoporotic trabecular and cortical bone often hinder the stable fixation of osteosynthesis materials, leading to instability at the fracture site and an increased risk of fixation failure [2]. Moreover, insufficient mechanical stability within the fracture callus can impede local angiogenesis, thereby disrupting the inflammatory response essential for fracture healing [3]. Additionally, an unstable fracture callus may induce excessive production of pro-inflammatory cytokines, such as tumor necrosis factor, which can suppress the proliferation and differentiation of mesenchymal stem cells (MSCs). This impairment in cellular activity negatively affects new bone formation, ultimately resulting in suboptimal healing outcomes [4]. Collectively, these factors contribute to delayed union, nonunion, or the development of pseudoarthrosis. Despite significant advancements in surgical approaches for musculoskeletal injuries, there remains a strong focus on delaying surgical interventions and exploring conservative treatment strategies. Regenerative medicine has emerged as a promising field in contemporary trauma care. Mesenchymal stem cells freshly isolated from adipose tissue are being utilized for tissue regeneration. These cells interact with their surrounding microenvironment to generate new progenitor cells and secrete exosomes rich in cytokines, growth factors, chemokines, and microRNAs, all of which facilitate tissue repair and restore biological functions.
For therapeutic applications, achieving high cell counts, minimizing in vitro culturing, and ensuring rapid processing are essential to maintaining efficacy. In orthopedic practice, the success of MSC-based therapies is directly dependent on the quantity of viable cells within the preparation, underscoring the need for rigorous verification of both qualitative and quantitative cellular parameters. The stromal-vascular fraction derived from adipose tissue serves as a primary cellular component, while autologous concentrated plasma (ACP) provides essential growth factors that facilitate tissue regeneration [5].
Case report
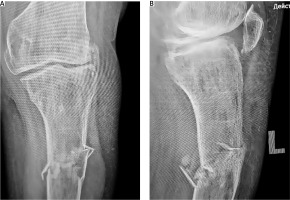
A 52-year-old female patient sustained a low-energy injury as a result of a fall from her own height, impacting the left lower limb. In the emergency department, the patient was assessed by an orthopedic specialist. Diagnostic evaluation included radiographic imaging to confirm the fracture and standard laboratory blood tests to evaluate general clinical parameters, performed in accordance with local treatment protocols. The vascular surgeon ruled out any damage to the peripheral blood vessels. A neurological assessment revealed no irregularities in the functioning of the peripheral nervous system. An X-ray examination of the left lower limb in two projections revealed a complex comminuted fracture of the proximal third of the tibia with fragment displacement (Fig. 1). Based on the findings, a diagnosis of a closed comminuted fracture of the proximal third of the left tibia was made. According to ICD-10 it was classified as S82.10, and according to the AO Müller system as 42-C3.
From the patient’s medical history, she has congenital anomaly of the left lower limb in the form of congenital absence of the fibula; four-toed left foot; shortening of the left lower limb by up to 8 cm; and ankylosis of the left ankle joint. Among the associated conditions, the presence of neurological pathology in the lower lumbar region should be noted, resulting from impaired biomechanics of posture and gait caused by congenital anomalies. At the age of 49 years, postmenopausal osteoporosis was diagnosed (M81.0. according to ICD-10). The diagnosis was confirmed following additional examination conducted due to a fracture of the distal metaepiphysis of the radius. The T-score for the AP spine (L1–L4) was –3.0, and for the femur total mean, it was –2.2. The patient was not initially treated with osteoporosis drugs, but followed preventive recommendations in the form of taking vitamin D3 at a dose of 4000 IU and calcium citrate at 4800 mg (equivalent to 1000 mg of elemental calcium) daily, with regular monitoring of vitamin D3 levels (25-hydroxyvitamin D) and ionized calcium in the blood every three months. It is noteworthy that the use of recommended drugs was not systematic. Specific osteoporosis therapy was prescribed in the form of denosumab at a dose of 60 mg every six months (administered once before the injury, and the next dose was delayed due to the current fracture).
Staged surgical interventions (Fig. 2):
Fig. 2
A) X-ray imaging of the injured tibia was performed 4 months after the initial application of external fixation, B) X-ray imaging of the injured limb after external fixation was remounted
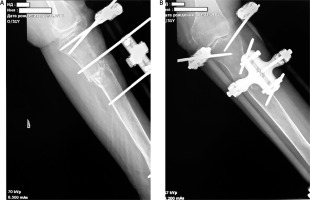
2 November 2022 – date of injury with installation of the primary external fixator;
18 November 2022 – first remounting of external fixation device due to instability of the rods, DXA examination;
December 2022 – secondary remounting of external fixation device due to instability of the rods;
March 2023 – the absence of X-ray signs of fracture consolidation. Installation of a compression-distraction device for controlled stimulation of osteogenesis;
21 June 2023 – absence of signs of fracture consolidation. DXA examination and Lower Extremity Functional Scale assessment. Injection of autologous MSCs into the fracture area;
12 July 2023 – first injection of growth factors in ACP into the fracture area;
2 August 2023 – second injection of growth factors in ACP into the fracture area;
29 September 2023 – radiographic signs of the beginning of the consolidation process. Removal of the external fixation device. The injured limb was immobilized with hard cast fixator. Lower Extremity Functional Scale assessment;
December 2023 – lower extremity functional scale assessment;
January 2024 – radiographic and clinical signs of final fracture consolidation.
FRAX assessment
Throughout the entire period of the established diagnosis, treatment was carried out in accordance with the state recommendations of Ukraine developed on the basis of European guidelines for the treatment of osteoporosis [6].
The following therapy was prescribed: zoledronic acid, 5 mg intravenously, administered once every 12 months; vitamin D3 at a dose of 4000 IU and calcium citrate at 4800 mg (equivalent to 1000 mg of elemental calcium) daily, with regular monitoring of vitamin D3 levels (25-hydroxyvitamin D) and ionized calcium in the blood every three months.
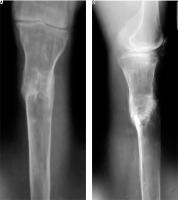
Eight months after the injury and prescribed therapy, the patient showed signs of delayed union of the fracture during follow-up X-ray examination, and therefore a decision to stimulate bone tissue regeneration by a single injection of MSCs into the fracture area, followed by maintenance injection therapy with growth factors into the fracture area, was made (Fig. 3). The total number of growth factor injections was 2, with a frequency of 3 weeks.
Fig. 3
X-ray examination of the left tibia, conducted 8 months after the start of treatment, reveals signs of delayed fracture healing
Mesenchymal stem cells were obtained from adipose tissue harvested from the anterior abdomen and prepared according to a standard protocol [7]. Written informed consent was obtained from the patient following a detailed explanation of the procedure and for publication of the next results. The patient received an injection into the fracture site using a 16G × 3½ needle, positioned between the fragments in a volume of 4 ml of plasma. All procedures were performed in a sterile operating theater under the supervision of a digital C-arm monitor. The injection was administered through a single approach to multiple points, involving needling and disruption of scarred soft tissue formations.
It should be noted that, against the background of the complex treatment of osteoporosis, an improvement in densitometry indicators was noted (Table 1).
Table 1
Comparison of the patient’s densitometry results – 2 weeks and 8 months after injury
To monitor bone mineral density (BMD), densitometry was performed using the General Electric Lunar Prodigy Primo device at two weeks and eight months after injury.
FRAX analysis was conducted at the end of treatment with subsequent comparison of the results to those obtained prior to the described specific anti-osteoporotic therapy treatment. It should be noted that between the diagnosis of postmenopausal osteoporosis and the current clinical case, there was a documented radial fracture (based on the patient’s history), which was already taken into account in the FRAX parameters.
According to the FRAX fracture risk assessment – which is based on several models, integrating clinical risk factors and femoral neck BMD – the risk of major osteoporotic fracture decreased 13–8.4 and the risk of hip fractures 6.2–2.3. Although a new fracture occurred during the study period, improvements in BMD and changes in certain clinical risk factors (e.g., reduction in steroid use, smoking cessation) ultimately outweighed the impact of this incident fracture, leading to a net decrease in the recalculated FRAX estimates.
Throughout the treatment period, the functioning of the injured lower limb was assessed using the Lower Extremity Functional Scale. Positive dynamics were noted: 8 months after injury – 37/80 = 46.3%; 11 months – 42/80 = 52.5%; 14 months – 44/80 = 55.0%. Three months into regenerative therapy, X-ray findings indicated the beginning of the consolidation process, evidenced by the formation of periosteal callus. Because of positive dynamics in treatment, the external fixation was replaced with a hard cast fixator.
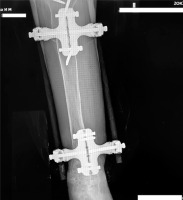
In the subsequent 3 months, X-ray signs of bone callus remodeling with final consolidation of the fracture are observed. Throughout the entire treatment period (from the moment of application of the compression-distraction fixator), the patient underwent rehabilitation under the individual supervision of an instructor (including axial loading exercises and work on the distal parts of the lower limb, etc.), with subsequent intensification after the removal of the hard cast, with constant monitoring of BMD and maintenance therapy for osteoporosis (Fig. 4).
Conclusions
Postmenopausal women face a higher risk of developing osteoporosis due to estrogen depletion, which reduces osteoprotegerin production and leads to an overall increase in bone turnover. Given the pathogenesis of osteoporosis and the age-related decline in the differentiation, activation, and function of osteogenic mesenchymal cells, it is reasonable to suggest that the processes involved in bone regeneration could be adversely affected by osteoporosis. Mesenchymal stem cells are essential contributors to bone formation. Mesenchymal stem cells-driven condensation is the initial step, followed by the differentiation of MSCs into chondrocytes during the formation of growth plates. These growth plates are subsequently replaced by new bone during longitudinal endochondral bone growth [8]. Recent studies have revealed that in patients with osteoporosis, MSCs are more likely to differentiate into adipocytes rather than osteoblasts, resulting in disruptions in bone formation [9, 10]. Mirsaidi et al. discovered that a single intratibial administration of isogeneic adipose-derived MSC (AD-MSCs) notably enhanced the quality of trabecular bone and led to a substantial rise in multiple molecular markers associated with bone turnover [11]. Furthermore, AD-MSCs contributed to an increase in BMD and stimulated new bone formation. These findings collectively highlight that AD-MSC transplantation is an effective cellular therapy for the treatment of osteoporosis of any etiology, including menopausal. By leveraging the understanding of MSC properties and the molecular mechanisms governing the differentiation of MSCs into osteoblasts and adipocytes, researchers achieved the desired outcomes by modifying MSCs through a combination of extracellular and intracellular factors.
This case report demonstrates that freshly isolated MSCs implanted directly into a bone fracture environment can stimulate de novo bone tissue. It should be noted that the mechanical effect of needling and destruction of connective tissue scars does not destroy it fully formed in the fracture area, but makes it porous with perforators, which creates conditions close to a “membrane scaffold” that holds the MSCs in the required concentration and protects them from contact with external cells, which increases their chance of differentiating into osteoblasts. Along with its cellular component, ACP releases several growth inducers:
vascular endothelial growth factor facilitates the growth and development of new vascular endothelial cells;
fibroblast growth factor promotes cell proliferation, collagen synthesis, and hyaluronic acid production;
transforming growth factor β AD-MSCs triggers angiogenesis, among other effects.
Of course, we should not underestimate the effect of the drug therapy for osteoporosis, which affects the mineral link of the process and provides the body with a reserve for bone tissue restoration, as well as physio-functional therapy, which through mechanical loading promotes targeted cell differentiation and restoration of bone architecture. In this case, MSC/ACP therapy acts as a trigger mechanism of an additional push when regeneration processes slow down.
Unfortunately, there are currently no extensive meta-analyses dedicated to the risks of using mesenchymal stromal cells and ACP therapy in patients with fractures. However, individual studies and reviews indicate potential risks associated with these methods. The use of MSCs and ACP therapy in patients with fractures presents both promising regenerative potential and notable risks. While these therapies aim to enhance bone healing through growth factors and cellular differentiation, concerns remain regarding immune responses, infection risks, and uncontrolled tissue formation. Mesenchymal stem cells may lead to heterotopic ossification, while ACP efficacy can vary depending on preparation methods and patient-specific factors. Additionally, standardized protocols and long-term safety data are still lacking, highlighting the need for further clinical trials to ensure the safe and effective application of these regenerative strategies in fracture management.
In summary, the following theses can be formulated: local injection therapy with MSCs and ACP is effective only as an element of multidisciplinary therapy in the treatment of complications of tubular bone fractures (e.g., delayed consolidation) in patients with systemic osteoporosis; regenerative therapy with MSCs and ACP growth factors serves as a supplementary approach for managing fracture complications in patients with menopausal osteoporosis prior to radical surgical intervention. This method promotes tissue proliferation by stimulating the body’s innate regenerative potential. The case report represents a promising start, but further research is needed to confirm the efficacy and safety of this therapy.