Introduction
Vitamin D is an essential fat-soluble vitamin that is obtained from dietary sources and unrestricted exposure to sunlight. The latter stimulates cutaneous synthesis of this essential vitamin from solar radiation. Measurement of circulating 25-hydroxyvitamin D [25(OH)D] is considered to be the gold standard test for vitamin D status in the body. Under most clinical settings, vitamin D insufficiency and deficiency are both diagnosed if serum 25(OH)D levels are found to be less than 30 ng/ml and 20 ng/ml, respectively. Vitamin D inadequacy adversely affects bone health due to secondary hyperparathyroidism, which excessively stimulates bone remodeling and causes fragility. As a result, vitamin D deficiency causes serious bone complications such as rickets, osteomalacia, osteoporosis, and fractures [1]. In addition to the adverse outcomes of vitamin D inadequacy on bones, several other systemic diseases have been linked to the deficiency of this vitamin, including autoimmune diseases, cancer, and cardiovascular diseases [2].
Although vitamin D deficiency is common among all age groups, it is especially prevalent among women after menopause, possibly due to poor exposure to sunlight and insufficient intake of vitamin D from diet [3]. Postmenopausal women who are vitamin D deficient are prone to develop serious complications such as osteoporosis and consequent catastrophic fractures of the neck of the femur and vertebral column [4]. The prevalence of vitamin D deficiency in postmenopausal women varies in different countries. Indeed, in the European Union, 32.1% of women after menopause were estimated to have 25(OH)D less than 20 ng/ml [5] whereas in India, China and the United States, the prevalence of vitamin D inadequacy was reported to be 53.3%, 72.1%, and 53%, respectively [6-8].
A significant debate exists regarding the most effective therapeutic regimen for correcting vitamin D deficiency in postmenopausal women. In this context, several clinical trials have been conducted to investigate the most appropriate dose, route of administration and duration of therapy with varying outcomes. In this systematic literature review, we aimed to draw conclusions on the most appropriate treatment protocol for vitamin D deficiency in this group of women.
Material and methods
We searched PubMed over the period 2000 to 2018 using the following search terms: “Vitamin D deficiency dose”, “Vitamin D deficiency supplement”, “Vitamin D deficiency supplementation”, “Vitamin D deficiency cholecalciferol”, and “Vitamin D deficiency cholecalciferol dose”. Studies were included if they were original articles, clinical or randomized clinical trials, published in English, conducted on vitamin D deficient but healthy postmenopausal women, using vitamin D3 (cholecalciferol). Manuscripts were excluded if they were review articles, cross-sectional studies, or conducted on animals or in vitro. Studies were also not included if the full article was not available or if vitamin D analogs, other than vitamin D3, were used. Articles were also eliminated if they were conducted on mixed populations such as men and women or involved mixed age groups, if the route of administration and duration of therapy were not clearly stated and if baseline and post-treatment 25(OH)D were not reported. Eligible manuscripts were initially reviewed by reading the title and abstract, but when the reviewers were not able to make a decision, the full article was reviewed. Eligible articles were identified by two independent reviewers. However, in the case of disagreements two additional reviewers were consulted. The outcome of interest in this review was the change in 25(OH)D levels after treatment with vitamin D3. Regimens reported in the included studies were considered effective if the post-treatment 25(OH)D level exceeded the cutoff point of 30 ng/ml.
Results
Search results
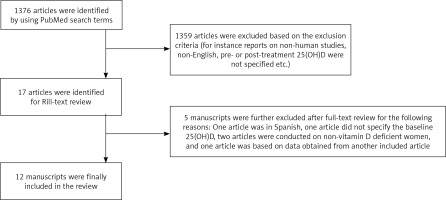
We identified 1376 articles which matched the search criteria (Fig. 1). Based on reviewing title and abstract, 1359 articles were excluded as per the exclusion criteria. The 17 articles which were eligible for full-text review were read in full by the investigators. Out of those, 5 manuscripts were further excluded as follows: One article was in Spanish, one article did not specify the baseline 25(OH)D, two articles were conducted on non-vitamin D deficient women, and one article was based on data obtained from another included article. The remaining 12 manuscripts were finally included (Table 1).
Table 1
Summary of included studies.
| Author, year | Postmenopausal women’s characteristics/Randomization/Study quality testing | Study design/Vitamin D3 regimen | Mean baseline and post-treatment 25(OH)D, ng/ml | Conclusions |
|---|---|---|---|---|
| Hansen, 2015 | N: 230 Age: 61 ±6 years Block randomization was performed Intent to treat was performed | Randomized clinical trial Loading dose of oral 50,000 IU/day for 15 days followed by maintenance dose of oral 50,000 IU every 2 weeks for 11.5 months | Baseline: 21 ±3 Post-treatment: 30 days: 80 60 days: 59 120 days: 46 240 days: 45 365 days: 42 | Oral loading dose of 50,000 IU/day for 2 weeks followed by 50,000 IU/2 weeks for a year was effective for raising 25(OH)D level above normala and was maintained up to 1 year Maintenance oral dose of 800 IU/day was not sufficient to raise serum 25(OH)D level to normal, p < 0.001b |
| Randomized clinical trial Maintenance dose of oral 800 IU/day for 12 months | Baseline: 21 ±3 Post-treatment: 30 days: 26 60 days: 27 120 days: 29 240 days: 28 365 days: 27 | |||
| Romagnoli, 2008 | N: 32 Age range: 66-97 years No information on randomization was stated No study quality testing was reported | Prospective randomized intervention study Loading oral dose of 300,000 IU | Baseline: 13.3 ±9.9 Post-treatment: 30 days: 47.8 ±7.3 60 days: 28.06 ±8.33 | A single oral dose of 300,000 IU had a faster onset and was more effective than equal intramuscular dose in elevating 25(OH)D to normal levels but after 60 days, neither doses were effective in keeping 25(OH)D level cbove normal, (p < 0.001c) |
| Prospective randomized intervention study Loading intramuscular dose of 300,000 IU | Baseline: 8.3 ±3.6 Post-treatment: 30 days: 15.91 ±11.3 60 days: 26.16 ±12.1 | |||
| Aloia, 2014 | N: 76 Age: 58 ±4.9 Computer randomization was Performed No study quality testing was reported | Randomized clinical trial Maintenance oral doses of 800, 2000, or 4000 IU/day for 2 months | Baseline: 25.2 ±5.6 Post-treatment: Mean at 800: 26 Mean at 2000: 32 Mean at 4000: 40 | Maintenance oral dose of 800 IU/day was not effective. Maintenance oral dose of 2000 IU/day successfully raised 25(OH)D to normal. Maintenance oral dose of 4000 IU/day produced the highest response (p = 0.002b) |
| Ceglia, 2016 | N: 21 Age: 78 ±5 No information on randomization was stated No study quality testing was reported | Randomized clinical trial Maintenance oral doses of 4000 IU/day for 4 months | Baseline: 17.6 ±4 Post-treatment: 32 ±4.8 | Maintenance oral dose of 4000 IU/day was effective for raising serum 25(OH)D above normal (p < 0.01d) |
| Golombick, 2008 | N: 23 Age: 61.2 ±3.2 No study quality testing was reported | Prospective open-label study Maintenance oral dose of 1000 IU/day for 1 month then 500 IU/day for 2 months | Baseline: 14.4 ±0.4 Post-treatment: 36 ±2.4 | Loading dose of oral 1000/day for one month followed by maintenance oral dose of 500 IU/day for one month raised serum 25(OH)D to normal in 86% of women (p = 0.0001d) |
| Cangussu, 2015 | N: 160 Age: 58.8 ±6.6 Computer randomization was performed Intent to treat was performed | Randomized clinical trial Oral 1000 IU/day for 9 months | Baseline: 15 ±7.5 Post-treatment: 27.5 ±10.4 | Maintenance oral dose of 1000 IU/day for 9 months was not sufficient to raise serum 25(OH)D to normal (p < 0.001d) |
| Thomas, 2010 | N: 22 Mean age: 66 Computer randomization was Performed No study quality testing was reported | Randomized clinical trial Oral 1000 IU/day for 7 weeks | Baseline: 19.6 Post-treatment: 30.8 ±7.2 | Maintenance oral dose of 1000 IU/day for 7-8 weeks was sufficient to raise serum 25(OH)D to normal (p < 0.001d) |
| Randomized clinical trial Oral 1000 IU/day for 8 weeks | Baseline: 19.6 Post-treatment: 36 ±10 | |||
| Gallagher, 2012 | N: 163 Age: 67 ±7.3 Computer randomization was performed No study quality testing was reported | Randomized clinical trial Oral 4800 IU/day for 12 months | Baseline: 15.3 ±3.7 Post-treatment: 46.5 | Maintenance oral dose of 4800 IU/day for 12 months was effective to raise serum 25(OH)D above normal |
| Mason, 2016 | N: 218 Age: 59.6 ±5.9 Block randomization was performed Intent to treat was performed | Randomized clinical trial Oral 2000 IU/day for 12 months | Baseline: 21.4 ±5.1 Post-treatment: 35 | Maintenance oral dose of 2000 IU/day for 12 months was sufficient to raise serum 25(OH)D above normal (p = 0.02d) |
| Talwar, 2007 | N: 208 Age: 59.9 ±6.2 Computer randomization was performed Intent to treat was performed | Randomized clinical trial Oral 800 IU/day for 24 months followed by oral 2000 IU/day for another 12 months | Baseline: 18.8 ±8.4 Post-treatment: 3 months: 28.4 ±8.8 24 months: 26.4 ±8.8 27 months: 34.8 ±10.8 36 months: 29.6 ±10.8 | Maintenance oral dose of 800 IU/day for 24 months was not sufficient to raise serum 25(OH)D to normal. Maintenance oral dose of 2000 IU/day for 12 months was effective to raise serum 25(OH)D above normal in 50% of women (p < 0.0001d) |
| Viljakainen, 2006 | N: 49 Age: 65-85 No information on randomization was stated No study quality testing was reported | Randomized clinical trial Oral 200, 400, or 800 IU/day for 3 months | Baseline: 18.8 ±6 Post-treatment: 200 IU/day: 23.2 ±3.6 400 IU/day: 24 ±3.6 At 800: 28.4 ±3.6 | Maintenance oral dose of 800 IU/day for 3 months failed to raise serum 25(OH)D above normal |
| Apaydin, 2018 | N: 60 Age range: 50-68 No information on randomization was stated No study quality testing was reported | Randomized clinical trial A single oral dose of 300,000 IU | Baseline: 9.7 ±4.4 Post-treatment: 1 month: 35.9 ±9.6 3 months: 23.1 ±4.7 | Maintenance oral dose of 800 IU/day for 3 months was not sufficient to raise serum 25(OH)D above normal (3.2% of treated women had repletion).A single oral dose of 300,000 IU was more effective (63.3% of women were repleted, p = 0.049c) but did not maintain 25(OH)D level above normal after 3 months of follow-up |
| Randomized clinical trial Oral 800 IU/day for 3 months | Baseline: 10.2 ±4.4 Post-treatment: 1 month: 16.9 ±5.8 3 months: 19.8 ±7.2 |
Review results
Vitamin D3 was administered orally in the majority of the included manuscripts. Indeed, only one study tested intramuscular vitamin D3, while the remaining 11 articles reported using the oral route. Regarding the tested doses and duration of treatment, most studies investigated low daily doses of vitamin D3 which ranged from 200 to 4800 IU/day for 2-12 months (9 studies). One study reported administering a single high dose of 300,000 IU while another 2 studies compared high doses which ranged from 50,000 to 300,000 IU with low daily doses.
Regarding the studies which tested high doses of vitamin D3, Romagnoli et al. described the outcomes of administering a single high dose of 300,000 IU orally or intramuscularly [9]. The oral dose successfully corrected 25(OH)D values. However, follow-up of vitamin D status demonstrated failure of this regimen to maintain 25(OH)D levels beyond sufficiency after 60 days. In comparison, an equal dose of vitamin D3 administered intramuscularly was not effective to correct vitamin D deficiency either after 30 days or after 60 days. Apaydin et al. reported the efficacy of administering an oral dose of 300,000 IU but adequate levels of 25(OH)D were not maintained after 3 months of follow-up [10]. Hanssen et al., on the other hand, reported treatment with loading doses of 50,000 IU/day daily for 2 weeks followed by the same dose every 2 weeks for one year [11]. Monitoring of the mean 25(OH)D levels showed that levels of 80 ng/ml after 4 weeks and mean levels above 30 ng/ml were maintained for one year.
For trials which investigated low daily doses of vitamin D3, five studies reported administering oral doses of 800 IU/day, but none of them revealed that this dose was effective in raising mean 25(OH)D above 30 ng/ml [10-14]. Three studies used an oral dose of 1000 IU/day, though the results were conflicting. Cangussu and his colleagues found that, after 9 months of continuous therapy with this dose, the 25(OH)D response was suboptimal [15]. However, Colombick et al. [16] and Thomas et al. [17] revealed mean values of more than 30 ng/ml after around 2 months of treatment with this dose. All the remaining studies reported regimens which examined doses beyond 2000 IU/day and all of them reported positive results. Indeed, Aloia et al. [12], Talwar et al. [13] and Mason et al. [18] tested administering an oral dose of 2000 IU/day for 2-12 months, and all of them reported an increase in mean 25(OH)D that was consistently above 30 ng/ml. Aloia et al. [12] and Ceglia et al. [19] reported using an oral dose of 4000 IU/day for 2 and 4 months, respectively. Both doses invariably elevated mean 25(OH)D above the sufficiency level. Lastly, Viljakainen and coworkers reported using an oral dose of 4800 IU/day for 1 year [14]. The investigators revealed that this dose resulted in an increase in the mean vitamin D levels to 46.5 ng/ml at the end of the treatment period.
Discussion
By conducting an extensive literature review, this study aimed to draw conclusions on the most effective regimen for correcting vitamin D deficiency in women after menopause using vitamin D3. For this purpose, we reviewed clinical trials conducted on vitamin D deficient, healthy postmenopausal women over the period 2000 to 2018. The reviewed articles described different regimens for correcting vitamin D deficiency which used variable doses, routes of administration and duration of therapy. Overall, our findings reported that daily maintenance doses ranging from 2000 to 4800 IU/day were the most effective and most commonly used in the included studies. Those regimens not only increased 25(OH)D to adequate levels but they consistently maintained it with continued therapy.
Our findings revealed that oral vitamin D was the most effective and most commonly used route of administration, compared to the intramuscular one which was employed occasionally and reported to be subtherapeutic in some of the studies. Indeed, Romagnoli et al. found that intramuscular administration of a single high dose of 300,000 IU of vitamin D3 failed to correct vitamin D deficiency compared to an equal dose that was administered orally [9]. In line with these data, Zabihiyeganeh et al., conducted a randomized clinical trial in which participants were administered 300,000 IU either orally or intramuscularly in six divided doses over three months. At the end of the treatment period, measurement of 25(OH)D levels revealed that the oral route was significantly more effective compared to the parenteral regimen [20]. In contrast with these data, however, Masood et al. reported that a single intramuscular dose of 600,000 IU of vitamin D3 was significantly more effective compared to an equal dose of orally administered dose and the levels of 25(OH)D levels remained higher at 6 months of follow up [21]. On the other hand, Wylon et al. reported that 8000 IU/day of oral vitamin D3 administered over 84 days was comparable to a single intramuscular dose of 100,000 IU in elevating 25(OH)D in vitamin D deficient adults [22]. Although conflicting results were reported on the efficacy of oral and intramuscular vitamin D3, our findings in postmenopausal women revealed that oral vitamin D3 was adequate to correct vitamin D deficiency.
When we reviewed the dose and frequency of administration of vitamin D3 which were used for supplementation, we found that the majority of included manuscripts reported using daily maintenance doses rather than a single high dose, or an initial high loading dose, followed by maintenance doses. Hackman and colleagues conducted a study on 59 vitamin deficient participants who were treated with either 50,000 IU/day for 10 days or 3000 IU daily for one month, followed by 1000 IU daily for another 2 months. The investigators concluded that both regimens were equally effective in correcting vitamin D deficiency [23]. Although small daily doses of vitamin D3 were used frequently for correcting vitamin D deficiency, this review revealed variability in the efficacy of different tested doses. Indeed, in all included articles, doses beyond 2000 IU/day were shown to be universally effective. On the other hand, while a dose of 1000 IU/day was reported to be inadequate in some studies, doses which ranged from 200 to 800 IU/day were universally ineffective as well. In line with this, ter Bor et al. conducted a study to examine the 25(OH)D response to 800 IU/day of vitamin D3. The researchers concluded that this dose produced suboptimal results in 50% of participants after 10 weeks of treatment [24]. Based on this review, we recommended using a vitamin D3 dose beyond 2000 IU/day for postmenopausal women who suffer from vitamin D deficiency. Our recommendations were in agreement with Chakhtoura et al., who conducted a systematic review and meta-analysis on vitamin D replacement in adults and older adults. They found that 2000 IU/day was the minimum effective dose to raise 25(OH)D to 20 ng/ml [25]. These recommendations are also in line with the latest guidelines of the United States Endocrine Society, which recommended daily doses of 1500-2000 IU/day for adults aged more than 19 years to keep 25(OH)D within the reference range [26].
Conclusions
Oral supplementation with vitamin D3 is adequate to correct vitamin D deficiency in postmenopausal women. Although daily vitamin D3 doses below 1000 IU/ day were not consistently shown to be effective, doses of 2000-4800 IU/day were reported to be adequate in all reviewed studies to reach the target 25(OH)D of 30 ng/ml.