Introduction
The liver is an organ that synthesizes many coagulation and anti-coagulation factors which play a pivotal role in maintaining the coagulation balance in our body. In patients with liver cirrhosis, thrombocytopenia and coagulopathy can appear and are associated with decreased liver function. Liver function is mostly assessed based on its ability to synthesize proteins, such as albumin, fibrinogen, proteins C and S, and other substances [1]. Liver cirrhosis causes changes in routine blood coagulation tests, which were thought to be associated with an elevated bleeding risk, such as increased international normalized ratio (INR) and activated partial thromboplastin time (aPTT) [2, 3].
Recent studies have shown that patients with liver cirrhosis have compensated coagulation, mostly due to the increased level of von Willebrand factor and factor VIII in the epithelium. Studies have also shown that this group of patients is not necessarily at greater risk for bleeding. On the contrary, thrombotic events were found to occur more commonly [2, 4]. These findings changed clinical practice regarding how patients with liver cirrhosis are managed and established thromboelastography (TEG) as a reliable alternative to routine coagulation blood tests [3]. Most studies have been conducted in patients with stable liver cirrhosis, and not many studies have evaluated coagulation using TEG in decompensated liver cirrhosis. Decompensated liver cirrhosis poses the greatest challenge in clinical practice, as bleeding events, severe coagulopathy, and thrombocytopenia occur more commonly.
Thromboelastography has not been previously used on a routine basis for the assessment of coagulation in patients with liver cirrhosis at our hospital. During our practice, we observed that, in most cases, BC transfusions were performed due to elevated INR, prolonged prothrombin time (PT), or low fibrinogen. Bleeding events caused by portal hypertension were mistakenly associated with changes in the previously mentioned laboratory values. Our main aim was to reduce BC transfusion by consulting practitioners based on TEG findings.
Material and methods
This was a prospective, single-center, randomized controlled trial. The research was conducted at Pauls Stradins Clinical University Hospital from May 2021 to May 2024. The study target group consisted of patients with decompensated liver cirrhosis, no history of active recent variceal bleeding. Patients were scheduled for invasive procedures with various bleeding risk categorized as low, moderate, or high. Bleeding risk was categorized according to the Consensus of Experts published in 2024 [5]. All procedures involving human participants adhered to the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained in accordance with Latvian regulations.
Our primary objective was to avoid unnecessary BC transfusions, such as fresh frozen plasma (FFP), cryoprecipitate (Cryo), or thrombocytes (platelets – PLT) in patients with decompensated liver cirrhosis by means of TEG. TEG was performed in all patients using the TEG 6s device (Haemonetics, Boston, MA). The test was conducted within 5 minutes of obtaining the blood specimen. After performing TEG, our team interpreted the results and consulted physicians regarding the degree of hemostatic alteration and the necessity of BC transfusion before invasive manipulations. Team members did not influence the subsequent decision-making of the treating physician regarding BC transfusions.
All team members were experienced gastroenterologists in terms of knowledge on rebalanced hemostasis and have completed online courses provided by Haemonetics. Decisions on prophylactic BC transfusions were made according to TEG results. If the reaction (R) time exceeded 9.1 minutes, administration of FFP at a dose of 10-15 ml/kg was recommended. If the citrated functional fibrinogen maximum amplitude (CFF MA) was below 15 mm, Cryo transfusion was suggested, and for citrated rapid TEG maximum amplitude (CRT MA) below 52 mm, PLT transfusion was advised.
Patients with an INR > 1.5 and/or a platelet count < 50 × 109/l were eligible for inclusion. All patients had decompensated liver cirrhosis, assessed using the Model for End-Stage Liver Disease (MELD) and the Child-Turcotte-Pugh (CTP) score.
The following exclusion criteria were applied:
severe infection (sepsis, as determined by quick-Sequential Organ Failure Assessment [qSOFA] clinical criteria and the Sequential Organ Failure Assessment [SOFA] score),
requirement for hemodynamic support with vasopressors,
pregnancy,
inability to provide informed consent for participation in the study,
use of anticoagulants or antiaggregants,
platelet count < 20 × 109/l,
ROTEM or other TEG method performed.
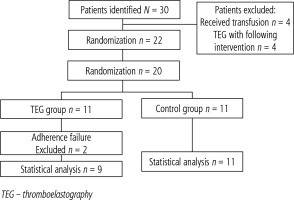
During the study, 30 potential patients were identified. In four cases, patients had already received BC transfusions for the correction of thrombocytopenia or coagulopathy assessed by using standard laboratory tests. In four additional cases, rotational thromboelastometry (ROTEM) had been performed by an intensive care unit physician prior to our consultation. A total of 22 patients were eligible for the study and provided written consent (Fig. 1).
Statistical data analysis was performed using IBM SPSS Statistics v. 28 software. Fisher’s exact test was used to evaluate transfusion reduction in the TEG and control arms. The Mann-Whitney U test was performed to analyze the association between INR, thrombocyte levels, and bleeding events.
Results
Twenty-two patients were included in the study. All patients were randomized and assigned to either the conventional treatment group or the TEG-guided transfusion group. After randomization, 13 (59.09%) patients were assigned to the TEG group, and 9 (40.91%) to the conventional treatment group. In both groups, the decision-regarding transfusion prior to the procedure was made by the treating physician and was not influenced by the research team.
In two cases (9.09%) – both in the TEG group – the decision not to perform an invasive procedure was made after patient enrollment. Therefore, statistical data analysis was performed on the 20 cases where an invasive procedure was carried out.
Of the 20 patients included in the study, gender distribution was equal, with 10 (50%) females and 10 (50%) males. The median age at admission was 47.3 years, with a median MELD-Na score of 21 and a minimum score of 7 points. Additional descriptive information can be found in Table 1.
Table 1
Descriptive data of cohort
In three cases (13.64%), both coagulopathy criteria were met, with an INR ≥ 1.5 and a thrombocyte count ≤ 50 × 109/l. Among those who met one coagulopathy criterion, 10 patients (45.45%) had an INR ≥ 1.5, while 7 patients (31.82%) had a thrombocyte count ≤ 50 × 109/l.
In two cases (22.22%) from the control group, bleeding occurred after the procedure, while no bleeding events were registered in the TEG group. In both cases, bleeding was managed successfully and did not have a significant effect on the patients’ general health. Bleeding occurred in one case after upper endoscopy with variceal band ligation and in the second case after central venous catheter placement.
After performing the Mann-Whitney U test, no significant association was found between thrombocyte levels and INR, with p-values of 0.80 and 0.089, respectively.
The distribution of high- and low-risk procedures was similar between the groups. In total, low-risk procedures were performed in 15 (75%) cases, and high-risk procedures were performed in 5 (25%) cases (Table 2).
Table 2
Procedure risk categorization between cohorts
In the TEG group, additional blood product transfusions were indicated in 4 patients (36.36%). In all four cases, TEG showed a CRT MA of less than 52 mm, and thrombocyte transfusion was performed. The median fibrinogen value in these patients was 1.98 g/l (1.48-4.6 g/l), and no additional correction of fibrinogen levels was performed. After consultation with the treating physician, transfusion was carried out in only one case (25%) despite observing changes in TEG results.
In the TEG group, two patients (18.18%) received transfusions despite normal TEG results. Among these patients, one received 12 doses of cryoprecipitate, while the second patient was given 11 doses of cryoprecipitate, 4 packs of thrombocytes, and 8 packs of fresh frozen plasma during admission (see Table 3). In the second case, the patient was diagnosed with infectious endocarditis, and transfusions were primarily performed during the perioperative stage.
Table 3
Characteristics of patients receiving transfusions despite normal thromboelastography (TEG) results
| Gender | Age (years) | MELD score | Tr (1 × 109/l) | INR | Fibrinogen (g/l) | Procedure performed | Cryo doses | Tr doses | FFP doses |
|---|---|---|---|---|---|---|---|---|---|
| Male | 61 | 19 | 72 | 1.68 | 1.14 | EVBL | 12 | 0 | 0 |
| Male | 35 | 35 | 36 | 1.92 | 1.70 | OHS | 11 | 4 | 8 |
To evaluate whether TEG reduced the need for transfusions, we used Fisher’s exact test to compare the control group with the TEG group. The analysis showed that when TEG was performed, the need for transfusions was only 20% lower than in the control group (p = 0.384).
We further hypothesized that all patients in the TEG group would have potentially received blood product transfusions prior to the procedure. Under this assumption, TEG would have reduced the need for transfusions by 69.2% (p > 0.05).
Discussion
In our study, we found that the use of TEG as well as education on the concept of ‘re-balancing’ hemostasis in patients with cirrhosis significantly reduces the likelihood of routine BC transfusions aimed at lowering bleeding risk or improving laboratory parameters (INR, aPTT, fibrinogen).
In a large nationwide study published by Desborough et al. in 2015, which included 1,313 patients with cirrhosis, it was found that one-third (30%) of patients received blood component transfusions during their hospitalization. Among those who received transfusions, 153 (39%) received them for the prophylaxis of bleeding, and 238 (61%) for the treatment of bleeding. Of the 238 patients, in 192 cases (81%), transfusions were administered for acute upper gastrointestinal bleeding [6]. The data provided by the authors emphasize the need for and importance of strict transfusion indication protocols for patients with cirrhosis. Recent advancements in the understanding of gastrointestinal bleeding have shifted the focus toward uncontrolled portal hypertension as the primary cause, rather than coagulopathy detected through routine blood tests. Evidence suggests that bleeding is often independent of conventional markers of coagulation abnormalities, underscoring the need for targeted management strategies addressing portal hypertension [7].
Another national survey, conducted in 2019 in Spain and including 135 professionals – 93 of whom were hepatologists – showed that the concept of hemostatic alterations and rebalancing in patients with cirrhosis was known to 74.8% of physicians.
During the survey, 23 physicians reported that they would correct INR prior to a low-risk procedure, 13 of whom were hepatologists. Thrombocyte levels below 50 × 109/l were reported as the most common indication for transfusion. Additionally, 39 physicians stated that they would perform transfusions if thrombocyte levels ranged from 26 to 50 × 109/l. Only one physician indicated that they would perform additional tests for hemostasis, such as TEG [8].
Thromboelastography has been shown to be an effective method for reducing the use of blood products in the management of thrombocytopenia and coagulopathy in patients with cirrhosis. In our study, we found that the use of TEG reduced the need for transfusions by 20%. It should be noted that our hospital does not have specific transfusion guidelines for patients with cirrhosis. All patients included in the study were treated by a hepatologist or gastroenterologist, which may partially explain why the effect of TEG on transfusion requirements was modest. In 2016, De Pietri et al. conducted a similar study and reported a greater effect of TEG, with a reduction in transfusions by 83.3%. The authors noted that 100% of the control group received transfusions based on hospital guidelines [9]. In our study, transfusions in the control group were based solely on the physicians’ discretion. Thrombocytopenia below 50 × 109/l is considered severe, and several studies have indicated an increased risk of bleeding even during low-risk procedures [7, 10]. As we reported, hypothetically, in a scenario where all patients in the control group received transfusions, the implementation of TEG would reduce the need for transfusions by 69.2%.
Napolitano et al. included 363 patients who underwent 852 procedures and found that thrombocytopenia and elevated INR did not predict bleeding after the procedure. The procedures performed included not only low-risk but also moderate- and high-risk interventions [11].
Our study has certain limitations, including a small sample size and its single-center design. However, as highlighted earlier, all patients were managed not only by gastroenterologists and hepatologists but also within a specialized ward of a university hospital. This setting strengthens the study, as it closely reflects real-world clinical practice in a highly specialized environment. Even in this context, where nearly all healthcare providers were familiar with the hemostatic alterations in cirrhosis, the use of TEG demonstrated a meaningful reduction in the need for transfusions.
Conclusions
In conclusion, our study demonstrated that the use of TEG meaningfully reduced the need for BC transfusions, even in a specialized university hospital setting where clinicians were well versed in the hemostatic alterations associated with cirrhosis. These findings underscore the potential of TEG to optimize BC transfusion practices in real-world clinical care.