Introduction
Metastatic renal cell carcinoma (RCC) is represented by 20–30% of all kidney cancers. After radical surgery, nearly one third of the localized RCC are expected to develop distant metastases [1].
Sunitinib malate is an oral multi-target tyrosine kinase inhibitor for both vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) receptors [2–4]. It was approved by the Food and Drug Administration (FDA) in 2006, then it has become the first line treatment for patients with metastatic RCC [5–7].
Sunitinib standard dose is 50 mg once/daily for 4 consecutive weeks followed by 2 weeks off to allow recovery from treatment related adverse events (TRAEs) (4/2 schedule) [8, 9]. Evidence has demonstrated that long-term and high exposure to it lead to severe adverse events (AEs), such as fatigue, hypertension, hand–foot syndrome (HFS), diarrhea and hematological toxicities [10].
In clinical practice, occurrence of AEs resulted in drug interruptions or dose reduction with a unfavorable impact on survival and patients quality of life [10, 11]. Therefore, many trials were done to find the best schedule that gives the best response with minimal toxicity, increased drug adherence, and enhanced overall time of treatment [3].
Some recent studies evaluated a modified two-week on and one-week off schedule (2/1 schedule) which showed improved clinical outcome and tolerability [12–16]. However, these studies were single-center and retrospective. So, this prospective multi-centric randomized trial was done to evaluate the standard 4/2 schedule vs. a modified schedule (2/1 schedule), then determine which schedule has superior efficacy, tolerability and clinical outcomes.
Material and methods
This prospective randomized multi-centric study included 70 patients with metastatic RCC who were operated, diagnosed, treated and followed up at; Urology Surgery, Medical Oncology, Clinical Oncology and Nuclear Medicine departments, Faculty of Medicine, Zagazig University, Egypt as well as Clinical Oncology Department, Assiut University, Egypt. The patients collected and followed up in the period from January 2017 to December 2019. All patients underwent nephrectomy except five patients diagnosed pathologically by needle biopsy; 3 patients in group 1 and 2 patients in group 2.
70 patients were randomly assigned into 2 groups:- 40 patients were allocated to group1 and given the standard dosing schedule of sunitinib of 50 mg per day, 4 weeks on and 2 weeks off (4/2 schedule) as reported by Motzer et al. [3]. The other 30 patients were admitted to group 2 where they received the alternative dosing schedule of 50 mg per day, 2-week on and 1-week off (2/1 schedule). Treatment was given until disease progression or unacceptable AEs.
In case of severe AEs and/or worsening intolerance, a decision of a dose reduction or stopping of sunitinib was taken by the physician.
All patients underwent initial radiological assessment by computed tomography (CT) chest, abdomen and pelvis Magnetic resonance imaging (MRI) brain and radionuclide bone scan, Positron emission tomography-computed tomography (PET-CT) when indicated, in addition to the laboratory investigations such as complete blood count (CBC), liver function test (LFT), renal function test (RFT), urine analysis, serum calcium. All patients underwent a regular follow up every 6 weeks starting from the end of the 4 weeks of sunitinib (schedule 4/2) and at the end of the second week of sunitinib (schedule 2/1).With each clinic visit, the overall and ≥ grade 3 incidence of treatment related adverse events (TRAEs) were assessed in each group of our study. A radiological evaluation by CT scan was done to assess the size of tumor every 3 months after starting sunitinib. The radiographic response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 [17]. Severe AEs were defined as those with grade ≥ 3 as classified by the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 [18]. All patients provided written informed consent. Our work was approved by the Ethical Committee of our institutions.
Patients were considered eligible when they: had histopathologically confirmed metastatic RCC (clear cell carcinoma and non-clear cell carcinoma), were aged ≥ 18 years, were treatment-naïve metastatic RCC patients, had clinical or radiological metastatic disease with normal organ and bone marrow function. Non-metastatic patients were excluded from the study.
The primary endpoint was to evaluate the treatment related AEs, while the secondary endpoints were evaluation of tumor response, progression-free survival (PFS) and overall survival (OS).
Statistical analysis
Data was tested for normal distribution using the Shapiro-Walk test. Categorical covariates were compared using the χ2 test or Fisher’s exact test. Mann-Whitney U test was used to calculate the difference between quantitative variables in the two groups. The OS and PFS were calculated by the Kaplan-Meier method and Survival curves were compared using the log-rank test. The Cox proportional hazards model was used for univariate analysis. Variables that were statistically significant in the univariate analysis were included in the multivariate Cox proportional hazards model. All tests were two-sided and a p-value ≤ 0.05 was considered statistically significant. All statistical analyses were performed using Statistical Package for Social Sciences (SPSS 24 Inc. Chicago, IL, USA). PFS was defined as the time from the initiation of sunitinib to progression of disease (PD) or death. OS was defined as the time from first administration of sunitinib till death from any cause or missed follow up.
Results
Table 1 shows the baseline characteristics of patients. Most of them were primarily males (58.6%). 47.1% of our patients were ≤ 60 years and 52.9% were > 60 years with median age 60 years (range, 42–68 years). Clear cell carcinoma was the most common pathological subtype (92.9%) of the patients. 58.6% of the patients had International Metastatic RCC Database Consortium (IMDC) intermediate-risk features, followed by (31.4%) had favorable IMDC risk features and 10% showed poor risk features. The majority of the patients had good performance status (PS) 0–1 about (92.9%). The most common metastatic sites were the lungs (48.5%), followed by LNs (20%), bone (15.7%), then liver (12.8%), and brain 2.8%. 47.1% of the patients had tumor size >10 cm. Positive lymph node (LN) was present in 65.8% of the patients. The majority of patients had nephrectomy surgery (92.9%). There were no significant differences between both groups regarding the patients characteristics. The median follow-up time was 23 months with a range of (12–34).
Table 1
Baseline characteristics of patients treated with sunitinib stratified by sunitinib dose schedules
Safety
Table 2 shows the incidence of AEs of sunitinib according to dosing schedule. All patients (100%) had AEs on schedule 4/2 vs. 73.3% on schedule 2/1 with significant difference between both groups (p = 0.001).Furthermore, patients who had grade 3 AEs on schedule 2/1 were significantly lower than patients on schedule 4/2 (26.7% vs. 82.5%), respectively, with (p < 0.001). There were significant differences between both groups regarding the fatigue (p = 0.002), thrombocytopenia (p = 0.015), diarrhea (p = 0.006), hypertension (p = 0.001), HFS (p = 0.001), mucositis (p = 0.001) and all were in the favor of group 2 (schedule 2/1) in comparison with group 1 (schedule 4/2). Regarding the incidence of grade 3 AEs, patients of group 2 (schedule 2/1) showed significantly less AEs than group 1 (schedule 4/2) such as fatigue, diarrhea, hypertension, HFS, mucositis (p < 0.001). The incidence of overall and grade 3 dyspepsia, hypothyroidism, anemia and leucopenia had no significant differences between both groups. Dose reduction was significantly decreased in 2/1 schedule group than 4/2 schedule group (13.3% vs. 95%) (p = 0.001).No one stopped treatment in the 2/1 schedule group vs. 31 patients (77.5%) in 4/2 schedule group (p < 0.001).
Table 2
Incidence of major adverse events according to the sunitinib dose schedules
Treatment efficacy and survival outcomes
As shown in Table 3, we analyzed the tumor response in both groups. The overall response was higher in group 2 (2/1 schedule group) than group 1(4/2 schedule group) (63.3% vs. 47.5%) with no statistical differences between both groups. Complete response (CR) was achieved in one patient (2.5%) of group 1 (4/2 schedule group) vs. 3 patients (10%) of group 2 (2/1 schedule group). A total of 34 (48.6%) patients had partial response (PR); 18 were in the group 1 (4/2 schedule), 16 were in group 2 (2/1 schedule). Seventeen (24.3%) patients had stable disease (SD); 11 patients (27.5%) were in group 1 (4/2 schedule) and 6 (20%) patients were in group 2 (2/1 schedule). Fifteen patients showed PD;10 were in group 1 (4/2 schedule),while 5 were in group 2 (2/1 schedule) with no statistical differences were observed between both groups. At time of the last follow-up, progression occurred in 32 (45.7%) patients; 21 (52.5%) patients were in group 1 (4/2 schedule), 11 (36.7%) were in group 2 (2/1 schedule) without any statistical differences between both groups. Death occurred in 45 (64.3%) patients; 26 (65%) patients in group 1 (4/2 schedule), 19 (63.3%) in group 2 (2/1 schedule) with no statistical differences between both groups.
Table 3
Outcome of patients as regard to the sunitinib dose schedules
We sub-analyzed and compared PFS and OS among patients with the two dosing schedules as in Table 4 and Figures 1–3. OS rate was insignificantly higher in group 2 (2/1 schedule) (18.8%) vs. (4.5%) in group 1 (4/2 schedule) (p < 0.639), with the median OS 24 ±0.6 months for group 1 and 25 ±0.7 months for group 2. Regarding PFS rate was significantly higher in group 2 (2/1 schedule) (60.9%) vs. (38.6%) in group 1 (4/2 schedule) (p = 0.008), with median PFS 16 ±0.4 months for group 1 but it was not reached in group 2.
Fig. 1
Kaplan-Meier curves illustrating the overall survival (OS) and progression-free survival (PFS) (months) in all patients
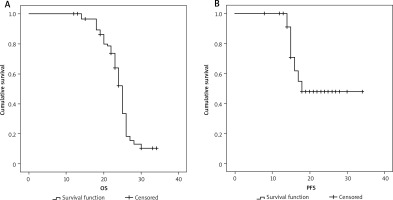
Fig. 2
Kaplan-Meier survival curves illustrating the overall survival rate differences (months) in group 1 vs. group 2
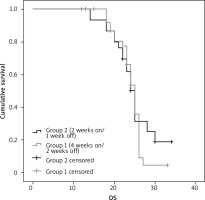
Fig. 3
Kaplan-Meier survival curves illustrating the progression-free survival rate differences (months) in group 1 vs. group 2
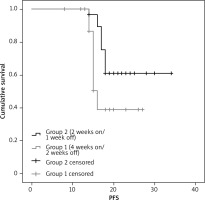
Table 4
The overall and progression-free survival rate in relation to the sunitinib dose schedules
We analyzed potential risk factors for PFS in patients with metastatic RCC treated with sunitinib (Table 5). By using Cox’s regression model, univariate analysis indicated that IMDC prognostic classification (poor risk category) (p < 0.037), ECOG performance status (≥ 2 vs. 0) (p < 0.001), tumor size > 10 cm (p < 0.004) and treatment schedule (group 1) (p < 0.018) were statistically associated with poor PFS.
Table 5
Outcome of patients as regard to the sunitinib dose schedules
Further multivariate analysis suggested that age > 60 years (hazard ratio [HR]) is 6.4, confidence interval (CI) [2.2–18.5], p = 0.001), IMDC prognostic classification (poor risk category) (HR 0.2 and CI [0.0–0.6], (p = 0.005), treatment schedule (group 1) (HR 0.2 and CI [0.1–0.6], (p = 0.003), were independent poor prognostic factors of PFS.
Discussion
Sunitinib is the principal treatment of patients with metastatic RCC as it was documented in many trials [3, 19, 20]. But it has many common adverse effects such as, diarrhea, fatigue, HFS, hypertension and thrombocytopenia, which are increasing throughout the cycle, and worsen most in the last two weeks of schedule 4/2; hence, it compromises the treatment continuation which result in interference with its therapeutic activity [3, 21, 22].
This raises the need to modify the strategy of administration of this drug to avoid drug discontinuation which could improve the outcome, decrease patients as well as their caregivers suffering, improve QOL of the patients; in addition, reduce the burden of health care providers [23, 24]. Therefore, we performed this study to compare both schedules of sunitinib administration in relation to the toxicity, efficacy and outcome. Reduction of the dose or stopping sunitinib was done for patients who received 4/2 schedule if they had severe AEs and/or worsening intolerance without doing shifting as it is not the aim of our study.
In our study, we found that the overall AEs were present in all patients (100%) on schedule 4/2 vs. 73.3% on schedule 2/1 with significant difference between both groups (p = 0.001) such as fatigue (p = 0.002), thrombocytopenia (p = 0.015), diarrhea (p = 0.006), hypertension (p = 0.001), HFS (p = 0.001) and mucositis (p = 0.001). All were in favor of group 2 (schedule 2/1) in comparison to group 1 (schedule 4/2). And the grade 3 AEs were statistically significant less experienced in patients on schedule 2/1 than the patients on schedule 4/2 (26.7% vs. 82.5%), respectively (p < 0.001) such as; fatigue, hypertension, HFS, mucositis, diarrhea (p < 0.001). The alternative 2/1 dose schedule had a significant decline of drug toxicity induced by dose reduction than 4/2 schedule group (13.3% vs. 95%) (p = 0.001) with no treatment interruption. It was noticed that the patients with poor ECOG PS has more worse toxicity and decreased tolerability in group 1 than group 2.
Our findings were consistent with Eldin [25] study which demonstrated that the alternative 2/1 schedule of sunitinib showed better toxicity profile compared to the traditional 4/2 schedule, with statistical significance for fatigue (p = 0.018), HFS (p = 0.008), mucositis (p = 0.010), hypertension (p = 0.038), diarrhea (p = 0.03), and thrombocytopenia (p = 0.023), because of dose reduction which was implemented due to drug toxicity. Neri et al. [15] conducted a phase II trial which showed improvement of the toxicity in 2/1 schedule group with a dose reduction occurred in only 9% patients which was in line with our study.
Our results were also in line with Karon study [26] who performed a single-arm, multicenter, phase 2 trial on 59 patients with metastatic RCC who received 2/1 schedule of sunitinib. They concluded that nearly one fourth of the patients had grade 3 fatigue, diarrhea or hand-foot syndrome, only 37% required dose reductions while 90% of patients continued treatment without stopping and avoided protracted high-grade toxicities.
But some studies examined the shifting from 4/2 schedule to 2/1 schedule (4/2-2/1), others compared it with the other two schedules (4/2 and 2/1 schedules) and checked if there were significant and intolerable adverse effects or not, e.g. Miyake et al. [27], Bracarda et al. [28] and Najjar et al. [14] who stated that switching from schedule 4/2 to schedule 2/1 lead to decrease the incidence and severity of sunitinib-induced toxicity. In addition, a significant reduction in toxicities (grade 3–4) was found in some trials such as fatigue, hypertension, hand–foot syndrome and thrombocytopenia [14, 28, 29]. These studies confirmed the results of other monocentric experiences [14–18]. Moreover, marked improvement of QOL was noticed [27].
Regarding the efficacy in our study, the overall response (OAR) was found to be insignificantly higher in 2/1 schedule than 4/2 schedule. This insignificance might be due to the small number of patients.
Eldin [25] and Zhang X et al. [28] found that the overall response (OAR) was not better in group 2 (2/1 schedule) which was consistent with our findings. They stated that this result was due to the small sample size.
In addition, Jonasch et al. study [23] showed that sunitinib (2/1 strategy) resulted in high efficacy, low rate of treatment discontinuation and maintained patients, QOL. Also, our findings were supported by Bracarda et al. [28] who stated that giving a 4/2–2/1 schedule did not impair efficacy. Furthermore, Karon [26] demonstrated that sunitinib showed high efficacy of 2/1 schedule with overall response rate 57%, with median PFS 13.7 months. However, they stated that the majority of the patients had intermediate risk (67%),while 10% of the patients had poor risk. Therefore, their non-randomized data supported the use of alternate 2/1 schedule to maintain QOL and increase duration of treatment.
In our trial, at the time of the last follow-up, progression was less in group 2 (2/1 schedule) than in group 1 (4/2 schedule) without any statistical differences between both groups. Death was not significantly higher in group 1 (4/2 schedule) than group 2 (2/1 schedule).
Regarding OS rate was insignificantly higher in group 2 (2/1 schedule) than in group 1 (4/2 schedule) (p = 0.639) with the median OS of 24±0.6 months for group 1 and 25 ±0.7 months for group 2. Regarding PFS rate was significantly higher in group 2 (2/1 schedule) than group 1 (4/2 schedule) (p = 0.008), with median PFS 16 ±0.4 months for group 1 but it wasn’t reached in group 2. The following criteria were considered independent poor prognostic factors of PFS by multivariate analysis;
age > 60 years (p = 0.001), IMDC prognostic classification (poor risk category) (p = 0.005), treatment schedule (group 1) (p = 0.003).
Our findings were in line with Eldin study [25] who found no difference in survival between both groups. However, the median PFS was 15 months and 17 months in groups 1 and 2, respectively and the median OS was 24 months and 23 months for groups 1 and 2, respectively.
Zhang X et al. [29] study demonstrated that the median PFS was longer in 4/2-2/1 schedule when compared with both the 2/1 and 4/2 groups (25.0, 11.0,12.5 months, respectively; p = 0.003). Similarly, OS was longer in patients in the 4/2–2/1 group than patients in the other two groups (median OS: 53.0, 28.0, 21 months, respectively; p = 0.03). However, the prognostic score (IMDC) ≥ 3 and a 4/2–2/1 schedule were considered as independent prognostic factors of PFS by multivariate analysis. In addition, nephrectomy was independent favorable prognostic factor (p = 0.002), while IMDC ≥ 3 was significantly associated with an increased mortality risk (p = 0.022). Furthermore, he stated that this treatment schedule may improve the clinical practice by giving personalized patient management and achieving individualized therapy using this schedule to patients with unfavorable IMDC risk category and higher tumor burden due to the survival improvement with 4/2-2/1 schedule. But this study had some limitations such as; it was a retrospective study with unavoidable biases of decision making and patient selection, limited medical records or reporting bias from physicians and patients resulting in bias of AEs evaluation, in addition inability to assess the patient compliance during treatment.
Furthermore, Bracarda et al. [28] stated, that there was no inferiority of the efficacy of 4/2–2/1 schedule regarding the survival as it showed longer PFS. They explained that by increasing drug exposure which was associated with increased efficacy and survival, as well as improved patients, clinical characteristics in the 4/2–2/1 schedule group than other patients groups. But it has some limitations such as its retrospective design, the use of a monocentric external control group, and the observational nature of the analysis. Also the small group of patients which started sunitinib on a 2/1 schedule, caused the small sample size and the negative selection bias of this group [28].
In addition, Britten et al. showed a survival benefits of 2/1 schedule and compared them with the worse outcome of the 4/2 schedule, which was in line with our results. They explained this result by the prolonged drug exposure [30].
Study limitations
Small sample size and doing our work on Egyptian patients only are considered limitations of our study, so further prospective studies with a larger number of patients with different ethnicities are needed to reach the final conclusion regarding the better dose schedule of sunitinib in metastatic RCC.








