Introduction
Thyroid cancer (TC) demonstrates steady growth in incidence worldwide and remains an urgent problem in oncology. Despite recent studies, some debatable issues about the volume of surgical intervention remain unsolved [1–3]. In 2020, 586,000 TC cases were detected worldwide (7.5 per 100,000). The incidence rates depend on sex (3 times higher in females than in males) and economical rate (higher in developed than in developing countries). The highest TC incidence was observed in North America, Australia, New Zealand, East Asia, and Southern Europe. The mortality rate has been relatively stable recently and reached 44,000 annual deaths in 2020 (0.4 per 100,000) [4].
Surgery is the primary treatment method for patients with TC. However, the adequate surgery volume for metastatic lesions of neck lymph nodes is currently being discussed. Surgical tactics range from avoiding the elective central lymph node dissection to total radical neck dissection [5–10]. This variation of surgical strategy is related to the risk of major postoperative complications and the controversial relationship between metastasis and mortality [11–14].
The detection of sentinel lymph nodes (SLN) with a selective dye and further histological examination aid in selecting the proper (personalized) surgical strategy and the volume of surgical intervention for clinically undetermined regional lymph nodes [15–17].
Toluidine blue is a cytochemical dye that has many applications in medicine. In the 1980s, this compound was used to assess damage to the anogenital area in sexual violence victims and to diagnose oral cavity neoplasms [18–21], and this diagnostic method is still relevant [22]. Toluidine blue is also used for diagnostics of endometrial pathology and during hysteroscopy [23]. This compound is also effective for the treatment of Alzheimer’s disease, leishmaniasis, and malaria diagnostic [24–26]. Moreover, toluidine blue is a known antidote used in patients with methemoglobinemia, which indicates the safety of intravenous and injection administration of this compound [27]. However, the histological staining of tissue sections remains the main field of its application [28].
A perfect dye should have high informativeness, low false-negative results, side effects, and anaphylaxis risk, and be available and cheap. Developed countries have a trend towards the combined use of blue dyes and radioisotope diagnostics. At the same time, low-income countries retain only blue dyes as the main interest for diagnostics [29, 30]. Despite the long history of application, there are no available data on the use of toluidine blue as a dye for SLN detection.
The purpose of the study is to evaluate the effectiveness and safety of intraoperative detection of SLN with a 1% toluidine blue aqueous solution. The significant tasks are to identify the pattern of TC metastases to cervical lymph nodes, to establish the prevalence of “skip” metastases, to compare the frequency of complications after total thyroidectomy and central neck dissection and lateral neck dissection with total thyroidectomy and central neck dissection, as well as to determine the feasibility of application of lateral cervical dissections in patients with papillary and follicular TC without metastases to regional lymph nodes (according to physical examination and ultrasound).
Material and methods
The ethics committee
The study was approved by the Ethics Committee of the Medical Institute of Sumy State University (Proceedings 2/7, 14 Jul 2021).
This research has been performed with the financial support of grants from the Ministry of Education and Science of Ukraine No. 0112U100471 “Condition of mineralized tissues using new composites with Ag+ and Cu2+ nanoparticles”.
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Medical Institute of Sumy State University (protocol 2/7, 14 Jul 2021).
Informed consent was obtained from all subjects involved in the study.
Data are available within the article.
Inclusion and exclusion criteria
A total of 187 patients with TC were selected for the study. Inclusion criteria were patients older than 18 years, cytologically confirmed papillary or follicular TC (cT1-4N0M0), no affected regional lymph nodes (according to physical examination and ultrasound), no history of allergy to food dyes, and no/compensated cardiovascular diseases.
Exclusion criteria were age under 18 years, anaplastic and medullary TC, enlarged lymph nodes of the neck during a physical examination and/or ultrasound, allergy to food dyes, severe cardiovascular pathology, active infection (AIDS, hepatitis, syphilis, tuberculosis), and the patient’s refusal to study participation.
Research design
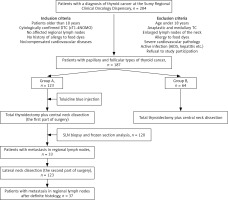
This is a prospective study. Patients were divided into 2 groups: group A (123 patients, 65.7%) did not have macroscopic metastases in the neck lymph nodes and underwent total thyroidectomy and central neck dissection, and lateral neck dissection; group B (64 patients, 34.3%) did not have macroscopic metastases to the neck lymph nodes and underwent total thyroidectomy and central neck dissection. The gender and age composition of both groups had no significant difference (p > 0.05). Patients were included in groups A and B according to criteria of similarity of age and gender. Patients were randomly selected for lateral neck dissection.
Preoperative evaluation
The histological type, disease stage, and condition of the regional lymphatic system were assessed before surgery.
Doppler ultrasound of the thyroid gland and all neck areas was performed with an expert-grade Philips N-Visor D-4 ultrasound machine (Netherlands) with a sensor frequency of 10 MHz in real-time. The dimensions, shape, and volume of thyroid tissue were assessed. All pathological formations in the thyroid gland and neck region were detected, and their localization, size, number of nodes, structure and the presence of pathological blood flow were assessed.
Fine-needle aspiration biopsy of the thyroid tumour was performed with a 25G needle under ultrasound control. At least 3 punctures were performed for each formation. Evaluation of all native cytological samples was carried out immediately after the puncture by May- Grünwald-Giemsa staining. Cytospins of sorted cellular populations were prepared by cytocentrifugation at 1000 rpm in a Shandon Cytospin 3 centrifuge for 5 min (Thermo Scientific, USA). The samples were fixed in methanol and stained with May-Grünwald stain for 15 min followed by 30 min in 5% Giemsa stain (Merck, Darmstadt, Germany). Slides were then washed with distilled water, dried, and mounted with Entellan (Merck, Darmstadt, Germany).
Surgical treatment
All interventions were performed at the Sumy Regional Oncological Hospital. During the operative treatment of group A patients, 1% toluidine blue was used as a dye for SLN visualization. Group B patients were not administered blue dye, and the biopsy of SLN was not performed. The selection of patients for the study and methods of treatment are presented in Figure 1.
Surgical treatment of group A patients
To perform the surgical intervention, the modified extended Kocher incision was applied (McVay modification). The front surface of the thyroid gland was initially exposed only in the tumour area to preserve lymphatic and venous pathways for better visualization of the regional lymphatic system. If the tumour was located no deeper than 1 cm from the front surface of the thyroid gland, an insulin syringe was used to inject toluidine blue solution. In cases of deeper tumour localization, a standard syringe with a 25G needle was used. The volume of the injected 1% toluidine blue aqueous solution corresponded to 10% of thyroid gland volume. After the injection, the puncture point was pressed with a gauze ball for one minute to prevent its leakage and staining of the surrounding tissue. Then, for 2–5 minutes, the outflow of the contrast through the lymphatic channels and the visualization of the regional lymphatic system was expected.
Operative treatment was carried out in 2 stages. In the first stage, a total thyroidectomy was performed with dissection of the central neck compartment (level VI), which included paratracheal, pretracheal, and prelaryngeal lymph nodes. After that, an urgent intraoperative frozen section histology examination of the thyroid tumour and SLN was performed.
In the second stage, lateral neck dissection was performed with the removal of tissue of the neck’s IIa, III, and IV levels, with mandatory revision of the Vb level. For further histological examination, all lymph nodes (sentinel and non-sentinel) were selected with a graphic representation on a mapping scheme of their size, number, and localization.
Surgical treatment of group B patients
Operative access by Kocher incision was used. No patients in this group were administered toluidine blue. A total thyroidectomy was performed with dissection of the central neck compartment (level VI), which included the removal of the paratracheal, pretracheal, and prelaryngeal lymph nodes. Urgent intraoperative histological examination of the thyroid tumour was performed. After surgery, all the removed material (thyroid with a tumour and tissue of level VI of the neck) was subjected to histological examination.
Morphological evaluation
Histology
Thyroid tumour tissue was fixed in a neutral (buffered) 4% formaldehyde solution for 24 hours, dehydrated, and saturated with paraffin. Paraffin blocks were sectioned with a thickness of 4 µm with a rotary microtome Shandon Finesse 325 (Thermo Scientific, USA). After deparaffinization and dehydration (with xylene and ethanol), histological sections were stained with haematoxylin and eosin.
Immunohistochemistry
Dehydrated sections were subjected to thermal unmasking of antigen in 0.1 M citrate buffer (pH 6.0) at 95–98°C (Thermo Scientific, USA). We used UltraVision Quanto Detec-tion System HRP and the DAB Quanto Detection System (Thermo Scientific, USA) for immunostaining and visualization. Sections were probed with monoclonal mouse anti-human thyroglobulin antibody (Tg) antibodies with a dilution of 1:200 (clone EPR9730, ab156008, Abcam, UK). Nuclei were counter-stained with Mayer’s haematoxylin. We used active (tissues with previously estimated positive and negative reactions) and passive (internal) control of immunohistochemistry (IHC).
All photos were captured with the digital visualization system based on Zeiss Primo Star microscope with a ZEISS Axio-cam ERc 5s digital camera and the software package “Zen 2.0” (Carl Zeiss, Germany).
Statistics
All data are summarized in a table, in which values are presented as averages and percentages. For continuous variables, the standard deviation is presented. The false-negative rate was defined as the percentage of metastatic neck lymph nodes missed after the use of toluidine blue. The sensitivity of the method was the ratio of the number of true positive results to the sum of true positive and false negative results. The specificity of the method was the ratio of the number of true negative results to the sum of true negative and false positive results.
The normality of data distribution was checked by the Shapiro-Wilk test. Student’s t-test was applied for the analysis of data with a normal distribution, and Mann- Whitney’s U test was applied for nonparametric data sets. The results were considered statistically significant with a probability of more than 95% (p < 0.05). Statistical analysis was performed in Microsoft Office Excel 2016 with AtteStat addon (version 12.0.5). All graphs were built in GraphPad Prism 9.
Results
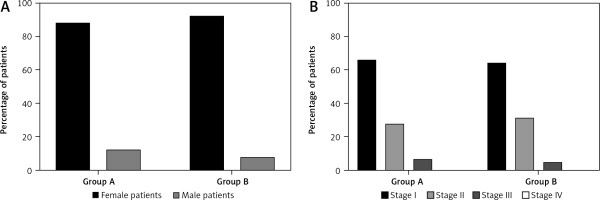
In total 187 patients with papillary and follicular TC and no signs of metastasis to regional lymph nodes (by physical examination and ultrasound) were selected for this study. Group A included 108 (87.8%) women and 15 (12.2%) men. The age of the patients ranged from 25 to 64 years (average – 46.3 ±1.54). Group B included 59 (92.1%) women and 5 (7.9%) men. The gender ratio was close to that of group A. The age of the patients ranged from 27 to 65 years (average 46.9 ±1.32) (Fig. 2 A).
The diagnoses of all patients were confirmed by cytology. The immunohistochemistry was applied for disputed cases. Papillary cancer was diagnosed in 105 (85.4%) individuals from group A and 56 (87.5%) from group B. Follicular cancer was diagnosed in 18 (14.6%) patients from group A and in 8 (12.5%) from group B.
The stage of the tumour was determined by the clinical and morphological classification of thyroid tumours according to the TNM system (8th edition, 2017). Among the patients of group A, the first stage of cancer was established in 81 (65.9%), and in the second and third stages in 34 (27.6%) and 8 (6.5%) people, respectively (Fig. 2 B). Among group B patients, the first stage of cancer was established in 41 (64.0%), and the second and third stages in 20 (31.3%) and 3 (4.7%) persons, respectively (Fig. 2 B).
During the first stage of surgery, all patients of group A underwent total thyroidectomy and central neck dissection. A 1% aqueous toluidine blue solution was used as a blue dye for the detection of SLN. Sentinel lymph nodes were visualized in 120 of 123 (97.6%) patients. The number of SLNs had a personal variation of 2–12 (4.2 on average). Based on the results of an urgent histological examination of frozen sections, metastases were detected in 33 of 120 (27.5%) patients (Table 1).
Table 1
The presence of metastases to the neck lymph nodes according to the intraoperative histological examination
In the second stage, all 123 patients of group A underwent lateral lymph node dissection followed by a final histological examination of sentinel and non-sentinel lymph nodes. In 33 (27.5%) patients, the results of the intraoperative and final histological examination completely coincided. In addition, 4 patients (IDs: 7; 12; 17; 28) were diagnosed with TC metastases only after the definite histological examination. Samples had a wide range of cytological features such as monolayered cell sheets (Fig. 3 F, 400×), nuclear pleomorphism, micropapillary structures of papillary fronds, colloid, foamy macrophages (Fig. 3 G, 1000×), giant multinucleated cells, intranuclear inclusions of macrophages (Fig. 3 G, 1000×), cytoplasmic vacuoles, and isolated cases of psammoma bodies.
Fig. 3
Metastatic papillary thyroid cancer in lymphatic node. Staining with haematoxylin-eosin (H&E) (A–C), immunohistochemistry detection of thyroglobulin (Tg) (D, E), cytological May-Grünwald-Giemsa staining (F, G)
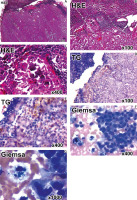
During the final histological examination of lymph node samples, significant changes in their structure were noticed. Small metastases were more often localized in the subcapsular zone of the lymph node. In some cases, the lymph node was replaced by tumour tissue represented by areas of solid and/or papillary growth of epithelial atypical tumour cells and with minor remnants of lymphatic tissue (Fig. 3 A, panorama 10×). Often, the TC metastases had a micropapillary structure and formed pseudo follicular structures (Fig. 3 B, magnification 100×). Another notable histological sign of TC metastases was the presence of rounded layered calcifications in the tumor tissue – the so-called psammoma bodies of various sizes (20–200 µm) (Fig. 3 C, magnification 400×).
In certain doubtful cases, the IHC with antibodies against thyroglobulin was applied to verify the histological origin of SLN metastases (Fig. 3 D, E).
Thus, SLN metastases were detected in 37 patients (30.8%). Therefore, the sensitivity of the sentinel lymph node detection with toluidine blue and further biopsy corresponded to 89.2% (33 out of 37 cases). The number of true negative results based on the final histological examination of SLN was 87. Five patients had false positive results from the intraoperative SLN examination. Accordingly, the specificity of the method corresponds to 94.6% (87 out of 92 cases). The positive predictive value was 88.03%, and the negative predictive value was 95.16%. The false-negative rate was 10.8%, and the false-positive rate was 5.4% (Table 2).
Table 2
Identification rate, sensitivity, specificity, positive predictive value, negative predictive value, false negative, and positive results of the sentinel lymph node biopsy technique
| Diagnostic test | Percentage |
|---|---|
| Identification rate | 97.6 |
| Sensitivity | 89.2 |
| Specificity | 94.6 |
| Positive predictive value | 88.03 |
| Negative predictive value | 95.16 |
| False negative results | 10.8 |
| False positive results | 5.4 |
The frequency of detection of metastases in lymph nodes at different levels of the neck is of significant clinical interest, which may indicate a certain regularity of TC metastasis.
The lateral neck dissection in patients of group A made it possible to assess the frequency of TC metastasis to the lymph nodes at different levels of the neck and to investigate the prevalence of skip metastasis. In most cases (83.7%), metastases were diagnosed in the central lymph node of the neck (levels VI and VII). In 64.8% of patients, lymph nodes of III, IV, and V levels were affected. Among them, exclusively level III lymph nodes were affected in 2 patients (5.4%). Quite rarely (5.4%) metastases were localized in the anterior-upper mediastinum (Table 3). The frequency of lesions of the central and lateral lymph nodes is shown in Table 4. The presence of metastases in the lateral lymphatic collector in the absence of metastases in the central lymphatic collector indicates the presence of skip metastases in a minority of patients. Therefore, skip metastases were detected in 6 (4.9%) patients.
Table 3
Frequency of lesions of lymph nodes on the neck in patients of group A
Table 4
Metastases in the central and lateral lymph nodes of the neck in patients after total thyroidectomy with central and lateral neck dissection. (+) – lymph nodes had metastases, (–) – lymph nodes did not have metastases
| Status of the central lymph node | Status of the lateral lymph node | Number of patients, n (%) |
|---|---|---|
| + | + | 18 (14.6) |
| + | – | 13 (10.6) |
| – | + | 6 (4.9) |
| – | – | 86 (69.9) |
Another aim of our study was to compare the frequency of postoperative complications in patients after total thyroidectomy with central neck dissection with lateral neck dissection (group A) and total thyroidectomy with central neck dissection (group B). Additionally, the safety of administration of toluidine blue as a blue dye was assessed.
Total thyroidectomy with central dissection, SLN biopsy, and lateral lymph node dissection (group A) was accompanied by the following complications: wound exudation (17 of 123 = 13.8%), lymphorrhagia (3 of 123 = 2.4%), wound inflammation (3 of 123 = 2.4%), transient vocal cord paresis (4 of 123 = 3.2%), permanent vocal cord paresis (1 of 123 = 0.8%), transient hypoparathyroidism (12 of 123 = 9.7%), and permanent hypoparathyroidism (2 out of 123 = 1.6%). None of the patients had an allergic reaction to toluidine blue.
Total thyroidectomy with central neck dissection (group B) was accompanied by the following complications: wound exudation (2 out of 64 = 3.1%), transient vocal cord paresis (3 out of 64 = 4.7%), transient hypoparathyroidism (8 out of 64 = 12.5%), and permanent hypoparathyroidism (3 out of 64 = 4.7%). In this group, no cases of lymphorrhagia, inflammation of the wound, or permanent paresis of the vocal cords were found (Table 5).
Table 5
Frequency of complications among patients of group A and group B
The most common postoperative complication in group A patients was the exudation of tissue fluid from the postoperative wound (seroma) – in 17 (13.8%) patients. The average time of exudation was 5.3 days (2–7 days). In 3 (2.4%) patients of this group, the cause of lymphorrhagia was an injury to the branches of the thoracic lymphatic duct of the neck. Treatment was carried out conservatively for 10–12 days. In group B patients, long-term exudation from the postoperative wound was seen in only 2 (3.1%) patients, and there was no lymphorrhagia. Inflammatory processes in the postoperative wound were diagnosed in 3 (2.4%) patients of group A because of prolonged lymphorrhagia. There were no such complications in group B patients. Postoperative unilateral vocal cord paresis occurred with the same frequency in patients of both groups. The transient paresis was seen in 4 (3.2%) patients in group A and 3 (4.7%) patients in group B. These complications passed without consequences in all patients of group B within 2 weeks. One (0.8%) patient from group A did not recover mobility of the vocal cord, which was considered permanent paresis.
The monitoring of the serum ionized calcium level was mandatory for all patients. The analysis was performed before the surgery and weekly during the postoperative period for a month. Transient hypocalcaemia was observed in 12 (9.7%) patients of group A and in 8 (12.5%) group B patients. Normalization of ionized calcium level occurred in 10 patients of group A and 5 patients of group B. Thus, the frequency of persistent hypocalcaemia was significantly higher in group A (4.7% vs. 1.6%).
Discussion
The most common blue dyes are isosulfan blue, patent blue V sodium, and methylene blue. Peek et al. showed that the average identification rate of SLN by using patent blue V was 83.2 ±10.3% (65–96%), methylene blue – 92.7 ±8.4% (83–98%), and isosulfan blue – 86.7 ±9.3% (73–98%). False-negative results of using patent blue V were 13.2 ±8.4% (4–23%), methylene blue – 6.4 ±8.2% (4–16%), and isosulfan blue – 13.3 ±2.0% (11–15%) [31].
Our research showed that toluidine blue was no less accurate than other blue dyes. The sensitivity of this method was 89.2%. False-negative results amounted to 10.8%. This value indicates the high effectiveness of the blue dye as a contrast agent. In comparison with other contrasts approved for use, only methylene blue has a higher value of this indicator [31]. In addition, no allergic reaction was recorded during the administration of toluidine blue. According to the MEDLINE and EMBASE databases, the incidence of allergic reactions to isosulfan blue and patent blue V ranges from 0.07 to 2.7%. At the same time, no case of allergy was recorded during the administration of methylene blue [32].
The standards of the National Comprehensive Cancer Network (NCCN) and the European Society of Clinical Oncology (ESMO) are usually followed in North American and European countries [33–34]. Lymph node dissection is performed only in cases of clinically detected metastases [35]. However, papillary TC is an independent risk factor for the presence of skip metastases [36, 37]. In turn, metastases in regional lymph nodes increase the risk of disease recurrence by 6.2 times and, as a result, lead to an increase in costs [38].
The frequency of skip metastases in patients with differentiated TC ranges from 4.2 to 19.7% [36, 39, 40]. In our study, this type of metastases was diagnosed in 4.9% of patients. The relationship between mortality and metastasis to regional lymph nodes is controversial; therefore, in general, we believe that with such a low prevalence of skip metastases, a lateral cervical dissection is appropriate only if metastases are detected by physical examination and/or ultrasound at the preoperative stage [41, 42].
It is generally accepted that lateral neck dissection increases the risk of temporary and permanent side effects. Rocke et al. in their global study of 55,204 patients reported that after unilateral neck resection, transient hypocalcaemia occurred in 20.3% and permanent hypocalcaemia in 4.7% of individuals [43]. In our study, the rates are much lower (9.7% and 1.6%, respectively). Intraoperative injection of toluidine blue allows better visualization of the regional lymphatic system and reduces the chance of accidental removal of the parathyroid glands. We believe that this is the reason for the significantly lower frequency of permanent hypoparathyroidism in group A patients (1.6% vs. 4.7%, respectively).
In a study by Rocke et al., vocal cord paresis was temporary in 3.3% of patients and permanent in 0.7% [43]. In our study, the frequency of this type of complication was comparable to the global study (3.2% and 0.8%, respectively). Among patients with lateral neck dissection, lymphorrhagia, inflammation, and wound exudation occur, which increases the duration of hospitalization and the economic costs of the treatment.
Conclusions
The proposed method of sentinel lymph node biopsy using toluidine blue dye is accurate, feasible, and safe in the detection of lymph node metastasis in the lateral neck compartment. We noticed no cases of an allergic reaction to the toluidine blue. The metastases in the central neck compartment (levels VI and VII) were diagnosed in 83.7% of patients. In 64.8% of patients, lymph nodes of III, IV, and V levels were affected. Among them, exclusively level III lymph nodes were affected in 2 patients (5.4%). Quite rarely (5.4%) metastases were localized in the anterior- upper mediastinum. Skip metastases were determined in 6 (4.9%) patients.
The incidence of complications after total thyroidectomy with central neck dissection and lateral neck dissection was higher compared with total thyroidectomy with central neck dissection. Thus, the use of the toluidine blue for intraoperative detection of sentinel lymph nodes with histological evaluation of their metastatic status allows to avoid unjustified neck dissections, and therefore avoid severe complications.
The low frequency of skip metastases (4.9%) and significant risk of postoperative complications (wound exudation, lymphorrhagia, inflammation, hypoparathyroidism, paresis of the vocal cords) support the idea that lateral neck dissection is appropriate only in case of confirmed metastases by physical examination and/or ultrasound at the preoperative stage.